Modular polyketide synthases (PKSs) such as the 6-deoxyerythronolide B synthase (DEBS) are a large family of polyfunctional, multi-subunit enzymes that catalyze the biosynthesis of structurally complex and medicinally important natural products known as polyketides (1, 2). Although polyketides are biosynthesized from simple acyl-CoA building blocks, their structural complexity often precludes the development of practical laboratory synthetic routes, leaving fermentation as the only viable source for the commercial production of these pharmaceutically and agriculturally useful agents. As the model system of modular PKSs, DEBS has been studied genetically and biochemically over the past two decades.
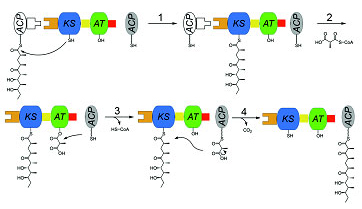
Similar to vertebrate fatty acid synthases, each DEBS module consists of at least two catalytic domains - a ketosynthase (KS) and an acyl transferase (AT) - that together collaborate with an acyl carrier protein (ACP) domain to catalyze polyketide chain elongation and inter-modular chain transfer (Figure 1). The modular nature of DEBS presents an attractive framework for engineering to generate novel medicinal polyketides (3). Therefore, it is essential to understand the structural and mechanistic features of DEBS.
Using x-ray diffraction data collected at SSRL Beam Line 11-1, Chaitan Khosla's group at Stanford University has determined the crystal structure of a 194 kDa homodimeric fragment of the KS-AT didomain of DEBS module 5 (Figure 2). This high resolution structure of the first didomain structure of a PKS module provides insights into the complex structural organization of the modular polyketide synthases machinery. The didomain structure was solved by multiwavelength anomalous dispersion techniques and has 40908 atoms (582 kDa) per asymmetric unit. To our knowledge this is the largest x-ray crystal structure that has been solved to date using the MAD technique. This project required the screening of around 600 crystals using SSRL's SAM robot.
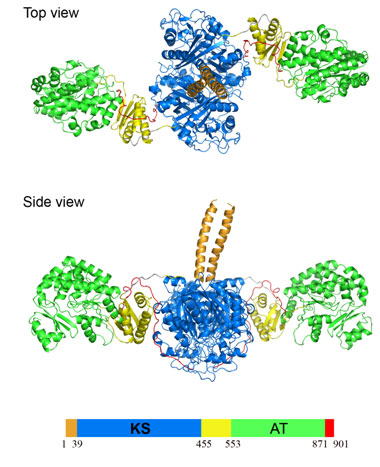
The 194 kDa homodimeric fragment contains full-length KS and AT domains as well as three flanking linkers: the N-terminal linker, an intervening KS-to-AT linker, and post-AT linker. The structure reveals that the KS domain adopts an abab fold, the AT domain contains an a, b-hydrolase-like core domain and an appended smaller ferredoxin-like subdomain. The three linkers are also structurally well defined. The N-terminal linker forms a coiled-coil structure, the KS-to-AT linker is formed by a three stranded b-sheet packed against two a-helices on one side, representing a protein fold not previously reported in the Protein Data Bank, the post-AT linker wraps back over both the AT domain and the KS-to-AT linker so as to interact specifically with the KS domain. Both the KS-to-AT linker and the post-AT linker play important structural roles in fixing the relative positions of the KS and AT didomain. The crystal structure also reveals that the active site Cys199 residue of the KS domain is more than 80 Å away from the active site Ser642 residue of the AT domain. This distance is too large to be covered simply by alternative positioning of statically anchored, fully extended phosphopantetheine arm of the ACP domain. Thus, substantial domain reorganization may be necessary for the ACP to interact successively with both the AT and the KS domains of this prototypical polyketide synthase module. These findings emphasize the critical role of unconserved but structurally well defined linkers as well as large interdomain movements in the structure and function of these remarkable modular megasynthases. The 2.7-Å KS-AT structure is fully consistent with a recently reported lower resolution, 4.5-Å model of fatty acid synthase structure (4), and emphasizes the close biochemical and structural similarity between polyketide synthase and fatty acid synthase enzymology.
In addition to providing the first atomic-level glimpses into the core catalytic domains of multi-modular PKSs, this prototypical structure also presents a fundamentally new perspective for future biochemical and engineering investigations into this remarkable family of modular megasynthases.
This work was supported by NIH grants CA 66736 and GM 22172
- Walsh, C. T. (2004) Polyketide and Nonribosomal Peptide Antibiotics: Modularity and Versatility. Science, 303, 1805-1810.
- Cane, D. E., Walsh, C. T., and Khosla, C. (1998) Harnessing the Biosynthetic Code: Combinations, Permutations, and Mutations. Science, 282, 63-68.
- Kumar, P., Khosla, C., and Tang, Y. (2004) Manipulation and Analysis of Polyketide Synthases. Methods Enzymol, 388, 269-293.
- Maier, I., Jenni, S. and Ban, N. (2006) Architecture of Mammalian Fatty Acid Synthase at 4.5 Å Resolution. Science, 311, 1258-1263.
Tang, Y., Kim, C.-Y., Mathews, I. I., Cane, D. E., Khosla, C. (2006) The 2.7Å Structure of a 194-kDa Homodimeric Fragment of the 6-Deoxyerythronolide B Synthase. PNAS, 103, 11124-11129.




