Manganese oxides are formed in soils, watersheds, and sea water via bacterial catalysis of the oxidation of dissolved Mn(II) to Mn(IV) (1). These remarkable but poorly understood oxides are relatively abundant in the environment (Mn is the second most abundant trace metal in the earths crust), where they are among the most aggressive scavengers of metal ions (1-3), and thus act as important sources and sinks for heavy metals. As a result Mn biooxides can exert a large influence over the trace metal chemistry of natural waters. For example, in acid-mine-drainage impacted streams, bacteriogenic Mn oxides commonly form coatings on cobbles and mineral grains in the stream and can naturally remove large amounts of heavy metal contaminants such as zinc from the water (4). Major scientific questions that come to the fore with respect to this issue are, "How (by what physical and chemical mechanisms) do Mn biooxides take up such high concentrations of metals?", and "How can we harness these processes to enhance the clean-up of metal-contaminated waters (in engineered remediation technologies, for example)?"
Uranium is a key contaminant of concern at US DOE sites and shuttered mining and ore processing locations around the united sites. Migration of uranium in ground water has contaminated drinking supplies in some locations (5,6) and threatens water supplies in other locations, leading to the need for costly clean-up activities. A major challenge to remediating ground water is that the contamination often resides at significant depth in the subsurface and is spread out over very large areas (hundreds of square meters to square kilometers). Subsurface remediation technologies, which are often designed to immobilize the metal contaminant of concern in place in the aquifer, therefore must utilize naturally existing reactants to produce products that are stable in the environment. Mn biooxides are of interest in this context because they may co-occur, or be stimulated to grow, in subsurface areas contaminated with uranium. However, the atomic-scale mechanisms by which they sequester uranium have received little attention.
In this study, the methods by which bacteriogenic Mn oxides sequester hexavalent uranium, U(VI), were investigated by a collaborative group of scientists from SSRL and the Oregon Health and Science University (7). Two complementary synchrotron based techniques were used to study these materials under conditions similar to those which may occur in the field: EXAFS (extended x-ray absorption fine structure) spectroscopy, which probes the short-range atomic structure (up to ~6 Å) within the materials. In-situ x-ray diffraction was used to probe long-range atomic order, including particle size and crystallinity.
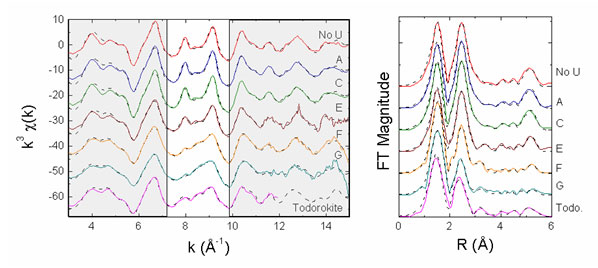
Mn K-edge EXAFS data are shown in Figure 2, where they are compared to the spectra for bacteriogenic Mn oxides ("No U" spectrum), which exhibit layered Mn-oxide structures, and the spectrum for a 3x3 tunnel-structure Mn oxide, todorokite ("Todo"). As can be seen in this figure, incorporation of U(VI) into Mn biooxides during oxide formation leads to a structure that is locally like todorokite, especially at the highest concentrations used. Fits to the spectra are consistent with the presence of a todorokite-like Mn oxide. At lowest U(VI) concentrations, fits to the spectra indicate a layered Mn oxide structure.
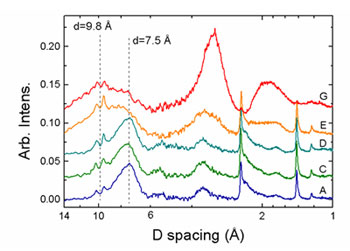
In-situ XRD results are shown in Figure 3. In samples A-D the patterns exhibit a broad 7.5 Å basal plane reflection and in-layer 2.45 and 1.40 Å peaks, as expected for layered Mn oxides. The breadth and low intensity of the basal peaks are typical of very small particle size as well as dispersion of the c axis repeat distance. The two small sharp peaks at 9.4 and 10.1 Å are due to diffraction from the bacterial cells. As the concentration of U(VI) increases (samples E and G), the intensity of the phyllomanganate basal plane reflection decreases and a new broad peak at 9.8 Å is observed, indicating the presence of a phase with long-range structure distinct from the layered Mn oxides. The shift from 7.5 Å to 9.8 Å occurs at sample E, which is the same region where EXAFS indicated that significant changes in the Mn local structure occurred. Spacings of 9.8 Å are exhibited by several manganates, including hydrated and expanded phyllomanganates as well as 3x3 tunnel structures, such as todorokite. This result is consistent with the Mn K-edge EXAFS result in that it suggests that the initial phyllomanganate Mn structure (low U(VI) concentration) was altered by U(VI) incorporation. XRD patterns from samples E and G also exhibit significant diffuse scattering located at 3.25 and 1.9 Å, which becomes more intense as U increases. Although it is difficult to assign structures that correlate to such a large, diffuse region of scattering intensity, the locations of the broad peaks are similar to reflections expected for todorokite. Diffraction simulations of todorokite with U(VI) present in the tunnels show that higher order peaks are enhanced over the intensity of the (001) reflection. Therefore, it is reasonable that these diffuse scattering peaks relate to the incorporation of U(VI) into the structure. The breadth of these peaks indicates a high degree of disorder, likely dominated by the fact that these particles may be aggregates of nanoparticles. Using the Scherer equation as a first-order approximation, a particle size estimate of 1.2 nm is obtained for sample G, suggesting that the cross-linked tunnel-like layers are essentially only one unit tall.
Fits to U LIII-edge EXAFS spectra (7) indicate that at lowest U(VI) concentrations, U(VI) is bonded to the surfaces and edges of the Mn oxides. At highest U(VI) concentrations, U(VI) is mostly bonded within tunnels in the Mn oxide structure. Altogether, these results indicate that, in the presence of U(VI), a transformation of the bacteriogenic Mn oxides occurs, with U(VI) being structurally bound within the resulting pseudo-tunnel nano-Mn oxide structures (Figure 4).
These findings are significant in the context of uranium transport in aquifers because they show that U(VI) may be structurally bound within bacteriogenic Mn oxides. Structural binding mechanisms provide a relatively high capacity to sorb the contaminant and simultaneously allow for slow release kinetics as compared to other modes of binding such as sorption at particle surfaces. These results indicate that bacteriogenic Mn oxides may be suitable for the long-term stabilization of subsurface U(VI) contamination in in-situ engineered remediation technologies.
This work was support by the National Science Foundation, Chemistry Division and by the US DOE, Office of Biological and Environmental Research, Environmental Remediation Sciences Program. This research was carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. DOE, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
- Tebo B. M., Bargar J. R., Clement B., Dick G., Murray K. J., Parker D., Verity R., and Webb S. (2004) Manganese biooxides: properties and mechanisms of formation. Annual Review of Earth and Planetary Science 32, 287-328.
- Villalobos M., Bargar J. R., and Sposito G. (2005a) Mechanisms of Pb(II) sorption on a biogenic maganese oxide. Environmental Science and Technology 39, 569-576.
- Villalobos M., Bargar J. R., and Sposito G. (2005b) Trace metal retention on biogenic manganese oxide nanoparticles. Elements 1, 223-226.
- Fuller C. C. and Harvey J. W. (2000) Reactive uptake of trace metals in the hyporheic zone of a mining-contaminated stream, Pinal Creek, Arizona. Environmental Science and Technology 34, 1150-1155.
- Board F. C. A. (1995) Fernald Citizens Task Force: Recommendations on Remediation Levels, Waste Disposition, Priorities, and Future Use. US. Department of Energy, Office of Environmental Management.
- Morris D. E., Allen P. G., Berg J. M., Chisholm-Brause C. J., Conradson S. D., Donohoe R. J., Hess N. J., Musgrave J. A., and Tait C. D. (1996) Speciation of uranium in Fernald soils by molecular spectroscopic methods: characterization of untreated soils. Environ. Sci. Technol. 30, 2322 -2331.
- Webb S. M., Fuller C. C., Tebo B. M., and Bargar J. R. (2006) Determination of uranyl incorporation into biogenic manganese oxides using x-ray absorption spectroscopy and scattering. Environmental Science and Technology 40, 771-777.




