Innate immunity is the front line host defense that acts within minutes of infection to counter invasion by microorganisms. Members of the Toll-like receptor (TLR) family recognize conserved pathogen-associated molecular patterns from virus, bacteria, fungi and parasites1, 2. In humans, at least 10 known TLRs are known to recognize different pathogenic molecular markers, such as viral double-stranded RNA (TLR3)3, flagellin (TLR5) and components of bacterial cell wall including lipopolysaccharide (LPS; TLR4) or lipopeptide (TLR2)4. Ligand-stimulated TLRs interact with various Toll/interleukin-1 receptor (TIR) domain containing adaptor molecules to activate signaling pathways that lead to a variety of immune responses and outcomes5.
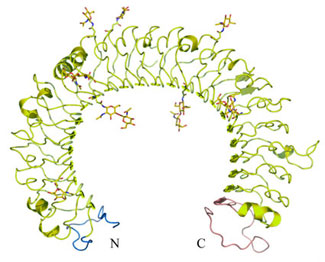
The structure of the human TLR3 ectodomain (ECD) was determined to 2.1 Å resolution using MAD data collected at SSRL Beam Line 11-1 and at the ALS, and represented the first structural look at any TLR ligand binding domain. The overall structure revealed a large horseshoe-shaped, right-handed solenoid structure comprised of 23 leucine-rich repeats (LRRs) (Figure 1). The inner concave surface is formed from 25 parallel b-strands, 23 from LRRs and one each from the N- and C-terminal cap region, that makes a highly curved, continuous b-sheet that spans 270o of arc. The outer convex surface contains an assortment of diverse secondary structural elements.
The LRRs of TLR3 (ECD) follow the typical consensus motif of a 24-residue repeat consisting of xL2xxL5xL7xxN10xL12xxL 15xxxxF20xxL23x, where L represents hydrophobic residues including leucine (most prevalent), isoleucine, valine, methionine and phenylalanine, F is a conserved phenylalanine, and N a conserved asparagine6. Seven conserved hydrophobic residues in this motif form a tight hydrophobic core of the solenoid structure and conserved asparagine at position 10 makes extensive hydrogen-bonding networks with its own and previous LRR motifs.
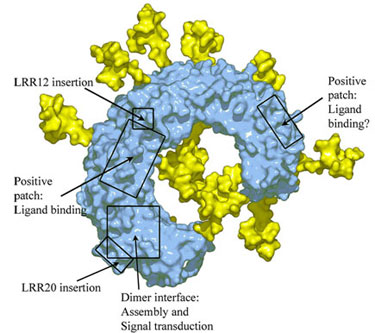
TLR3 ECD has 15 potential glycosylation sites and electron density for carbohydrate is observed for 8 of these sites. When oligomannans are modeled into all 15 sites, this reveals that most of the ECD surface, with the exception of one side face, is covered with carbohydrates. Contrary to the common belief that the inner concave space contains a ligand binding site, the inner concave surface of TLR3 has two glycosylation sites and many negatively charged residues that make it an unlikely binding site for dsRNA.
The glycosylation-free face contains two surface patches with a dense cluster of positively charged residues and a TLR3-specific insertion in LRR12 that could play a role in dsRNA binding. This face also contains a highly-conserved surface patch that coincides with a putative homodimer interface observed in the crystal and another TLR3-specific insertion (LRR20) that participates in the dimer interaction (Figure 2). Based on the location of glycosylation sites, the electrostatic surface potential, the TLR3-specific insertion and the dimer formation, we have proposed a model for the dsRNA binding site and mode of signal transduction.
- Beutler, B. Innate immunity: an overview. Mol Immunol 40, 845-59 (2004).
- Janeway, C.A., Jr. & Medzhitov, R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol 10, 349-50 (1998).
- Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732-8 (2001).
- Akira, S. Mammalian Toll-like receptors. Curr Opin Immunol 15, 5-11 (2003).
- Dunne, A. & O'Neill, L.A. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett 579, 3330-5 (2005).
- Bell, J.K. et al. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol 24, 528-33 (2003).
Choe, J., Kelker, M.S., Wilson, I.A. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science 309, 581-5 (2005).




