High-valent Fe(IV) and Fe(V) intermediates are invoked in the catalytic mechanisms of both heme and non-heme iron enzymes.[1, 2] Over the last several years, many Fe(IV)-oxo complexes have been synthesized and spectroscopically characterized, providing important markers for the characterization of Fe(IV) species.[3] In contrast, much less is known about Fe(V) species.
Recently, Frank Neese, Karl Wieghardt and co-workers have examined an Fe(V)-nitrido complex, which is generated by photolysis of an Fe(III)-azide precursor.[4] Mössbauer parameters for the Fe-nitrido complex are consistent with the an Fe(V) oxidation state assignment. However, further experimental data were needed to corroborate this result. For this reason, Fe K-edge X-ray Absorption (XAS) spectroscopy was used, in conjunction with electronic structure calculations, to obtain further insight into the Fe(V) complex.
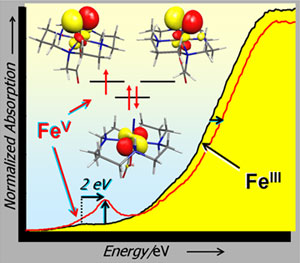
The Fe K-edge is sensitive to changes in the effective nuclear charge on the metal, showing an ~1 eV increase in the edge per oxidation state. Comparison of the Fe(V)-nitrido complex to the Fe(III) precursor shows an ~2 eV shift, consistent with the formulation of this complex as an Fe(V) species. The appearance of the pre-edge feature at 7114.2 eV (using a reference calibration point of 7111.2 eV for the first inflection point of an Fe foil) is ~1 eV higher than that of reported Fe(IV) species and provides an important reference for future XAS characterization of Fe(V). In addition, there is a dramatic increase in pre-edge intensity, consistent with the formation of a much shorter Fe-N bond in the nitrido species. EXAFS data were used to determine the first shell distances for the Fe(III) precursor and the Fe(V)-nitrido complex. It was found that the Fe(V) complex contained a short 1.60 Å Fe-N component, which was not present in the precursor.
Density functional theory (DFT) calculations on the Fe(V) species were carried out, allowing for either a doublet (S=1/2) or quartet (S=3/2) ground state. As the Fe(V) species is d3 and isoelectronic with Cr(III) and Mn(IV), an S=3/2 ground state would be expected. Surprisingly, the results favor an unprecedented doublet ground state. In addition, the calculations show a rather large difference in the short Fe-N bond distance, giving optimized Fe-N bond distances of 1.61 Å and 1.74 Å for the doublet and quartet states, respectively. Hence the computational results, coupled with the EXAFS analysis, support a doublet ground state in the Fe(V) species.
The combined experimental and computational results provide strong evidence for the formulation of the Fe-nitrido complex as a genuine S=1/2 Fe(V) complex. This study also provides important experimental markers for the characterization of other Fe(V) species, including high-valent iron intermediates within protein systems.
- L. D. Slep, F. Neese, Angew. Chem. Int. Ed. 2003, 42, 2942 and references therein.
- a) M. Costas, M. P. Mehn, M. P. Jensen, L. Que Jr., Chem. Rev. 2004, 104, 939; b) D. L. Harris, Curr. Op. Chem. Biol. 2001, 5, 724.
- a) J.-U. Rohde, J.-H. In, M. H. Lim, W. W. Brennessel, M. R. Bukowski, A. Stubna, E. Münck, W. Nam, L. Que Jr., Science 2003, 299, 1037; b) A. Decker, J.-U. Rhode, L. Que, Jr., E. I. Solomon, J. Am. Chem. Soc. 2004, 126, 5378.
- N. Aliaga-Alcalde, S. DeBeer George, B. Mienert, E. Bill, K. Wieghardt, F. Neese, Angew. Chem. Int. Ed. 2005, 44, 2908-2912.
N. Aliaga-Alcalde, S. DeBeer George, B. Mienert, E. Bill, K. Wieghardt, F. Neese, Angew. Chem. Int. Ed. 2005, 44, 2908-2912.




