Technetium (99Tc) is an abundant fission product that presents a number of challenges for the safe, long-term disposal of high-level nuclear waste due to the 213,000 year half-life of 99 Tc and the high environmental mobility of pertechnetate, TcO 4-, the most stable form of technetium under aerobic conditions. Because of these properties, 99 Tc is often the radionuclide of greatest concern when evaluating the near-term performance of waste repositories.
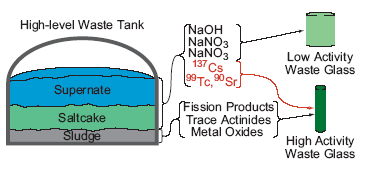
This concern is reflected in the initial plan for the vitrification of high-level nuclear waste stored in underground tanks at the Hanford site (Figure 1), in which the tank waste is separated into a high activity waste stream, consisting mainly of insoluble components, and a low activity waste stream, which is mainly the solution phase and includes most of the 99Tc in the waste tanks.1The high activity waste stream will be vitrified and the resulting glass will be sent to the high-level waste repository (currently Yucca Mountain). The low activity waste stream will be vitrified and the resulting low activity waste glass will be interred at the Hanford site. Because of the initially calculated rate of technetium release from the low activity waste glass, approximately 80% of the 99Tc in the solution phase needed to be separated and sent to the high activity waste stream.
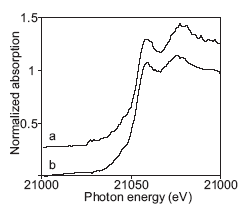
Initially, TcO 4- was believed to be the only technetium species present in the waste tanks, and separations based upon ion exchange of TcO 4- were developed. While ion exchange works well for many tanks, work by Schroeder, et al. (1995) showed that ion exchange could not remove a large fraction, up to 90%, of the technetium in certain tanks that contained a high concentration of organic complexants.2 The failure of ion exchange to remove the99 Tc was attributed to the presence of a soluble, non-pertechnetate species. A series of Tc K-edge X-ray Absorption Near Edge Structure (XANES) studies was carried out at SSRL by Blanchard, et al. strongly supported this explanation. 3 The XANES spectra (Figure 2) clearly showed the presence of a non-pertechnetate species in waste from tanks SY-101, SY-103, and AN-107; however, the identity of the non-pertechnetate species could not be determined from the XANES spectra.
Identification of the non-pertechnetate species is important for developing separation strategies for removing it from the low activity waste stream. Knowing its identity is necessary to determine which ion exchange material is appropriate, and identifying the non-pertechnetate species would allow the preparation of tank waste surrogates for laboratory testing of the separation strategies. One goal of our research was to identify which technetium species are stable in the highly alkaline environment of the Hanford high level waste tanks. The ultimate objective was to identify the actual non-pertechnetate species.
To better understand which technetium species might be formed in Hanford waste tanks, 1M NaOH solutions of TcO4- containing organic compounds that might be present in Hanford tanks were radiolytically reduced.4 The resulting compounds were examined at SSRL using beamlines 11-2 and 4-1. In almost all cases, only insoluble TcO2·2H2O was formed. On the other hand, when alkoxide ligands were present, soluble, red Tc(IV) complexes were formed. Both the insoluble TcO2·2H2O and the soluble Tc(IV) complexes were characterized by extended X-ray absorption fine structure (EXAFS). However, the alkoxides examined in the study are not present in sufficient concentrations to account for the presence of soluble, non-pertechnetate species. One compound found in Hanford tanks, gluconate, can potentially act as an alkoxide ligand with multiple binding sites and can form soluble Tc(IV) complexes in alkaline solution. The Tc(IV) gluconate complex was therefore examined by EXAFS.6 Although gluconate forms a very hydrolytically stable complex with Tc(IV), the XANES spectrum of the Tc(IV) gluconate complex is different from that of the non-pertechnetate species reported by Blanchard, et al.
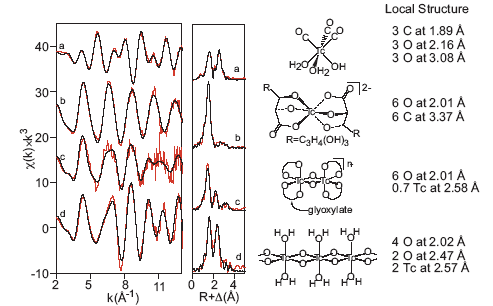
Since this approach did not appear to help identify the non-pertechnetate species, the XANES spectra of a variety of technetium species were calculated using FEFF85 and compared with the actual spectrum of the non-pertechnetate species. Surprisingly, the complex with the most similar calculated spectrum was the Tc(I) carbonyl complex fac-Tc(CO)3(H2O)3+. 6 Tc(I) carbonyl forms a stable, neutral complex, fac-Tc(CO)3(H2O)2(OH) in alkaline solution7 and also forms a stable complex with gluconate. The measured EXAFS spectrum of fac-Tc(CO)3(H2O)2(OH) is shown in Figure 3 along with the spectra of the Tc(IV) species that are stable in alkaline solution.
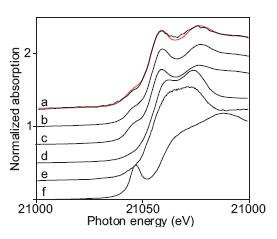
The XANES spectra of several technetium complexes are shown in Figure 4 along with the spectrum of the actual non-pertechnetate species. The XANES spectra of the Tc(I) carbonyl complexes are clearly very similar to that of the non-pertechnetate species. However, the identity of the non-pertechnetate species cannot be assigned to a particular Tc(I) carbonyl complex on the basis of their XANES spectra. The XANES spectra of both fac-Tc(CO)3(H2O)2(OH) and fac-Tc(CO)3(gluconate) fit the XANES spectrum of the non-pertechnetate species equally well. In addition, closely related Tc(I) nitrosyl complexes such as cis-Tc(CO)2(NO)(H2O)32+also exist and could be formed under the conditions present in Hanford waste tanks.8 The XANES spectra of these Tc(I) nitrosyl complexes would be similar to those of the Tc(I) carbonyl species. Consequently, although the XANES spectra of the non-pertechnetate species obtained by Blanchard and coworkers cannot be assigned to a specific technetium complex, the spectra can be assigned to a Tc(I) carbonyl or carbonyl/nitrosyl complex. These results indicate that a successful technetium separations technology must be formulated to target Tc(I)-carbonyl species.
Acknowledgement: This work was supported by the Environmental Management Science Program of the Office of Science and Technology of the U. S. Department of Energy (DOE) and was performed at the Lawrence Berkeley National Laboratory and the Los Alamos National Laboratory, which are operated by the University of California for the U.S. DOE under Contracts DE-AC03-76SF00098 and W-7405-ENG-36, respectively. Part of this work was performed at the Stanford Synchrotron Radiation Laboratory, which is operated by the Director, U. S. DOE, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences.
- "Response to Requirement for Report to Congress Under Floyd D. Spence National Defense Authorization Act for Fiscal Year 2001," Office of River Protection, 2000.
- Schroeder, N. C.; Radzinski, S. D.; Ball, J. R.; Ashley, K. R.; Cobb, S. L.; Cutrell, B.; Whitener, G. "Technetium Partioning for the Hanford Tank Waste Remediation System: Anion Exchange Studies for Partioning Technetium from Synthetic DSSF and DSS Simulants and Actual Hanford Waste (101-SY and 103-SY) Using ReillexTM -HPQ Resin.," LA-UR-95-4440, Los Alamos National Laboratory, 1995.
- Blanchard, D. L.; Brown, G. N.; Conradson, S. D.; Fadeff, S. K.; Golcar, G. R.; Hess, N. J.; Klinger, G. S.; Kurath, D. E. "Technetium in Alkaline, High-Salt, Radioactive Tank Waste Supernate: Preliminary Characterization and Removal," PNNL-11386, Pacific Northwest National Laboratory, 1997.
- Lukens, W. W.; Bucher, J. J.; Edelstein, N. M.; Shuh, D. K. Env. Sci. Tech. 2002, 36, 1124.
- Ankudinov, A. L.; Ravel, B.; Rehr, J. J.; Conradson, S. D. Phys. Rev. B 1998, 58, 7565.
- Lukens, W. W.; Shuh, D. K.; Schroeder, N. C.; Ashley, K. R. Env. Sci. Tech. 2004, 38, 229.
- Gorshkov, N. I.; Lumpov, A. A.; Miroslavov, A. E.; Suglubov, D. N. Radiochemistry 2000,43, 231.
- Rattat, D.; Schubiger, A. P.; Berke, H. G.; Schmalle, H.; Alberto, R. Cancer Biotherapy and Radiopharmaceuticals 2001, 16, 339.
Lukens, W. W.; Shuh, D. K.; Schroeder, N. C.; Ashley, K. R. Env. Sci. Tech. 2004, 38, 229.




