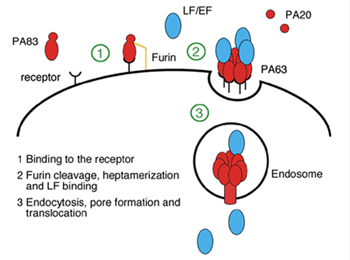
Infection by Bacillus anthracis, the causative agent of anthrax, involves the action of a secreted three-component toxin thought to disrupt host immune defences. Two of these components, lethal factor (LF) and oedema factor (EF) are enzymes that interfere with normal pathways inside the cell. The third, named protective antigen (PA), translocates LF and EF through the cell membrane into the cytosol1. In the initial stages of the intoxication mechanism (Fig. 1a), full-length PA (PA83) binds opportunistically to one of two cellular receptors (capillary morphogenesis protein (CMG2) or tumor endothelial marker (TEM8))2,3 and is proteolytically cleaved by a surface protease, furin, to a shorter polypeptide (PA63) that spontaneously heptamerizes to form a so-called pre-pore. Heptamers in turn are able to bind LF and/or EF and to trigger endocytosis of the receptor-bound toxin. Low pH conditions (around pH 5.5) then induce a pre-pore to pore conformational switch that allows the enzymes to enter and eventually kill the host cell. In severe cases, such as the inhalation form of the disease, this action proves fatal for the host. Further interest in this process is highlighted by the potential medical application of the toxin in treating certain cancers4. The structure of PA bound to one of its receptors, capillary morphogenesis protein 2 (CMG2) provides a picture of the very first stage of the mechanism of action of anthrax toxin (Fig. 1b).

Isolated PA83 is a 735-residue polypeptide comprising four domains (I-IV)5: domain I contains the furin cleavage site and prevents premature oligomerization of the full-length molecule; domain II is proposed to undergo the largest conformational change upon pore conversion and to provide the membrane spanning domain of the pore; domain III is involved in mediating intersubunit interactions in the heptamer; domain IV is responsible for receptor interaction (Fig. 1b). CMG2 contains an N-terminal 180-residue region similar to integrin A/I domains and therefore belongs to a large family of proteins used by cells to interact with their extracellular environment6. A/I domains bind their physiological ligands through a Metal Ion Dependent Adhesion Site (MIDAS) located at its upper surface: an acidic side chain from the ligand can complete the coordination sphere of the metal ion providing a large interaction energy.
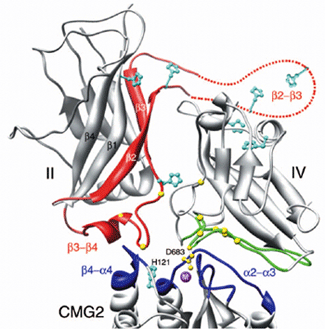
The structure of the complex was solved to 2.5 Å using data collected at beamline 9.1 at SSRL. Both proteins are largely unchanged upon complex formation. Asp683 of PA binds the MIDAS site, thus mimicking the mechanism of binding by physiological ligands. A large additional interaction surface provided by domain IV explains in part the ligand specificity of PA through shape complementarily. Unexpectedly, domain II was also found to interact with the receptor and to recognize a hydrophobic cleft, with a histidine (His 121) at its center, that appears to be unique to PA receptors among A/I domains, contributing further binding energy and specificity. Importantly, this interaction involves a short α-helix from domain II that is directly involved in the conformational changes leading to pore formation at low pH (Fig. 2). Therefore, it can be hypothesized that the receptor functions in stabilizing the pre-pore conformation of PA and therefore preventing premature conversion of the pre-pore to pore, which has been seen to occur efficiently at pH 7.0 in solution, but not on the surface of cells7. Furthermore, it has been pointed out that titration of histidine residues at low pH, several of which are located at or near the interface between domains II and IV (see fig. 2 and legend), could be implicated in the mechanism of pore formation5, and the present work shows that His 121, present in both receptors and located at the domain II binding interface, could serve a similar purpose.
A molecular dissection of the details of toxin action brings us closer to both the development of a therapy against anthrax infection and its use as a cancer therapeutic. In the latter respect, one immediate goal is engineering selectivity of PA towards its other receptor, tumor endothelial marker 8 (TEM8), which is predominantly expressed on the surface of cancer cells. In the former, direct structure based drug design will benefit from the new knowledge. In both areas we are moving towards a rational understanding that will guide the future directions of research in this area.
- Collier RJ, Young JA "Anthrax toxin" Annu Rev Cell Dev Biol 19, 45-70
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA "Identification of the cellular receptor for anthrax toxin" Nature 414, 225-9 (2001)
- Scobie HM, Rainey GJ, Bradley KA, Young JA "Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor" Proc Natl Acad Sci USA 100, 5170-4 (2003)
- Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH "Potent antitumor activity of a urokinase-activated engineered anthrax toxin" Proc Natl Acad Sci USA 100, 657-62 (2003)
- Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC "Crystal structure of the anthrax toxin protective antigen" Nature 385, 833-8 (1997)
- Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ "Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor" Proc Natl Acad Sci USA 101, 6367-72 (2004)
- Miller CJ, Elliott JL, Collier RJ "Anthrax protective antigen: prepore-to-pore conversion"Biochemistry 38, 10432-41 (1999)
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE "UCSF Chimera - A Visualization System for Exploratory Research and Analysis" J Comput Chem 25, 1605-1612 (2004)
Santelli E, Bankston LA, Leppla SH, Liddington RC "Crystal structure of a complex between anthrax toxin and its host cell receptor" Nature 430, 905-8 (2004)




