Cytochromes P450 are thiolate-ligated heme enzymes that play critical roles in a number of important physiological processes. P450s are involved in the metabolism of carcinogens and pesticides and in the production of glucocorticoids and sex hormones. Defects in these enzymes have been associated with disease states, such as glaucoma, congenital adrenal hyperplasia, psuedovitamin D-deficiency rickets, and Parkinson's.
Interest in P450s stems not only from their obvious biological importance but also from a desire to harness their synthetic potential. P450s catalyze the hydroxylation of organic substrates, often with high degrees of regio and stereo selectivity. When uncatalyzed, these reactions require extremely high temperatures to proceed even non-specifically. A recent x-ray absorption study performed at SSRL, hints at what allows the enzymes to perform these demanding reactions without destroying themselves.
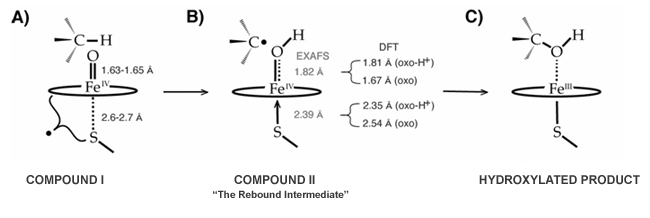
The reactive intermediates in P450s are thought to be Fe(IV)=O radical species, called compound I. Since these ferryl-radicals have proven to be elusive in P450s, Michael Green and coworkers examined the high-valent forms of chloroperoxidase (CPO), an oxidative fungal-protein with a similar thiolate-ligated heme in its active site. Using EXAFS spectroscopy, they determined that the Fe-O bond in the ferryl form of CPO is too long to be an authentic doubly bound iron-oxo species. The 1.82 Å Fe-O bond determined for this species is indicative of a protonated oxo, and it agrees well with density functional calculations on an Fe(IV)-OH species (1.81 Å). Green's experiments reveal that the ferryl form of CPO is basic, pKa > 8.2. The importance of this finding lies in its implications for P450 chemistry.
Peroxidases generally oxidize substrates by one-electron rather than two-electron processes. CPO is an exception. It is rather poor at one-electron oxidations, consistently oxidizing one-electron donors ten to a thousand times more slowly than typical peroxidases. Although CPO is a poor peroxidase, it readily performs two-electron oxidations: indeed, in its ability to hydroxylate hydrocarbons selectively, it functions more like a P450 than a peroxidase.
In the consensus hydroxylation mechanism (Figure 1), P450 compound I abstracts hydrogen from substrate to form a protonated ferryl (similar to the species found in chloroperoxidase), which subsequently hydroxylates the substrate radical. The ability of metal-oxos to abstract hydrogen scales with the strength of the O-H bond formed, D(O-H), during H-atom abstraction. This energy is determined by the reduction potential of compound I and the pKa of the ferryl species, as in Eq. 1.
D(O-H) = - 23.06 * E0cmpd-I- 1.37 * pKaferryl- 57 ± 2 (kcal/mol) (1)
This equation and Green's EXAFS results suggest a novel role for thiolate-ligation in cytochromes P450, namely, that Nature may be using the basic ferryl species afforded by thiolate-ligation to lower the redox potential necessary for hydrogen abstraction (and subsequent hydroxylation) to occur. In this way Nature can create a high-valent iron-oxo intermediate reactive enough to hydroxylate substrates as inert as cyclohexane without oxidizing the relatively fragile protein superstructure in the process.
M. T Green, J. H Dawson and H. B Gray, "Oxoiron(IV) in Chloroperoxidase Compound II Is Basic: Implications for P450 Chemistry", Science 304, 1653 (2004)

