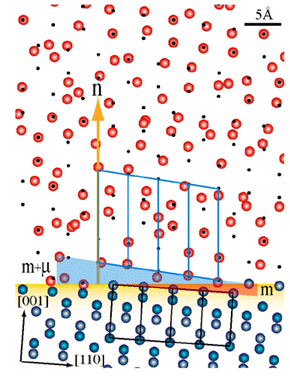
 ) plane. The silicon atoms in the substrate are blue and those in the oxide are red. The small black spots represent the translated silicon positions in the absence of static disorder. The silicon atoms in the oxide have been randomly assigned a magnitude and direction based on the static disorder value at that position in the lattice. The outline of four silicon unit cells is shown in black, whereas the outline of four expanded lattice cells in the oxide is shown in blue
) plane. The silicon atoms in the substrate are blue and those in the oxide are red. The small black spots represent the translated silicon positions in the absence of static disorder. The silicon atoms in the oxide have been randomly assigned a magnitude and direction based on the static disorder value at that position in the lattice. The outline of four silicon unit cells is shown in black, whereas the outline of four expanded lattice cells in the oxide is shown in blueOne of the most studied devices of modern technology is the field-effect transistor, which is the basis for most integrated circuits. At its heart it consists of two very different kinds of materials. The channel through which the electrical current flows is doped single crystal silicon, a semiconductor. The gate, which controls the current, is made of amorphous silicon dioxide, an insulator. The amplification of the transistor occurs because a small charge on the gate causes a large change in the conductivity of the channel. Ironically, these two materials started out as the same, single crystal silicon. It is through the thermal oxidation of the silicon that an amorphous oxide is formed, and until recently there was no evidence that the oxide had any "memory" of its former, crystalline self.
This has changed with the publication of work through a collaboration between researchers in industry and SSRL who found that there are weak crystalline "echoes" of the silicon's former self buried within the non-crystalline oxide. These "echoes" show up when probed using x-ray scattering in the form of additional diffraction peaks. To understand the cause of these peaks one must first understand how thermal oxidation occurs. There are many approaches for thermal oxidation in different atmospheres (O2, steam, etc.) but in every case the oxygen atoms diffuse into the silicon lattice and disrupt the lattice during the diffusion process, breaking the Si-Si bonds and forming Si-O bonds instead. One can get a sense of the expansion of the lattice between crystalline silicon and amorphous oxide by considering the volume per silicon atom in each case, 20.0 Å3 for crystalline silicon and 44.5 Å3 for amorphous SiO2. If, during the expansion process, the silicon atoms had remained in their atomic positions in the plane, and merely expanded their interatomic distances along the direction of the surface, then the new unit cell would be of tetragonal symmetry (which imposes two sides equal, one side longer, all three angles equal to 90 degrees) with a cell dimension in the growth direction of 44.5/20 or 2.23 times the former size. Due to the reciprocal nature of x-ray scattering, the resulting diffraction peaks would be located at 20/44.5 or 0.45 of their former positions along the surface direction.
This is, in fact, where the residual scattering is found. The specific oxidation method used has an effect on the amount of expansion of the silicon lattice, so the peak position is not always at 0.45, but is close to it. Also, because the oxidation dramatically increases the disorder of the silicon atoms in the oxide, the diffraction peak is one hundred thousand times weaker than what a comparable diffraction peak from crystalline silicon would be. It is only through the use of an intense synchrotron X-ray beam from SPEAR that the researchers were able to observe the residual order. The oxygen atoms in the oxide are completely disordered and do not contribute to the residual-order scatter peak. An atomic-scale view of the thermal oxide would look completely amorphous. This is demonstrated in the accompanying figure, which is a model of the atoms near the crystal-oxide interface using the same expansion and disorder parameters that were used to calculate the scattering observed for one of the samples. The black dots are the calculated positions of the silicon atoms in the oxide if only expansion occurred in the oxidation process. The actual positions are those after which both expansion and disorder have been taken into account. It is hard to believe from the figure, that atoms in these positions have residual long-range order.
In addition to confirming the residual order within the oxide, the shape of the diffraction peaks can be analyzed to determine specifics of the oxidation process. An asymmetry observed in the peak is due to a smaller lattice expansion near the interface than in the bulk of the oxide. This result is in agreement with studies by photoemission spectroscopy, which have identified sub-stoichiometric oxides at the interface. The disorder of the silicon atoms is also smaller at the interface, asymptotically growing to a final value far from the interface.
The model proposed in this study is general and has also been applied to studies of silicon surfaces along a number of different crystallographic directions, including one with a surface tilted 4 degrees from a principle direction. Although the scattering is different for each crystal orientation because the expansion is along a different crystallographic direction, in every case, the silicon atoms in the oxide have a "memory" of the original crystalline structure.
Analysis of oxide films can be used to determine the density of the oxide film as a function of depth. For the films that have been studied the oxidation recipe affects the density profile and in some cases the difference between surface and interface density is small. One exciting prospect emerging from the results of this study is if the amount of residual order can be related to the electronic properties of the oxide.
A. Munkholm and S. Brennan, "Ordering in Thermally Oxidized Silicon", Phys. Rev. Lett.93, 036106 (2004)




