Mammalian carboxylesterases are promiscuous enzymes responsible for processing a wide variety of drugs and xenobiotics (Redinbo et al., 2003). In particular, carboxylesterases metabolize and detoxify the dangerous narcotics heroin and cocaine, and the potent chemical weapon agents Sarin, Soman and Tabun. We have published the first three crystallographical studies of this distinctive group of enzymes and proposed detailed structure-function relationships. These findings have implications for drug design, the treatment of cocaine and heroin abuse, and victims of chemical warfare or terrorism. Beam Lines 7-1, 9-1 and 9-2 at Stanford Synchrotron Radiation Laboratory (SSRL) played a significant role in the data collection necessary to assemble these biological insights.
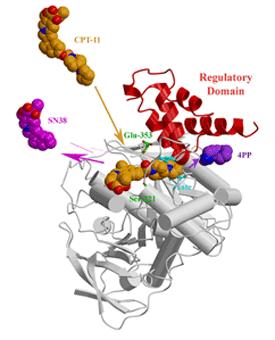
First, the structure of a mammalian carboxylesterase, that of rabbit liver carboxylesterase (rCE), reveals the activation mechanism of the camptothecin anticancer drug, irinotecan (CPT-11) (Bencharit et al., 2002). Using the position of a bound product on the surface of the enzyme, we have proposed that rCE utilizes a "side door" secondary product exit pore to facilitate the efficient processing of irinotecan (Fig 1).
Second, our work on human carboxylesterase 1(hCE1), the human homolog of rCE, are the first structures of a human enzyme bound to cocaine and heroin analogues (Fig 2a-b) (Bencharit et al., 2003a). The hCE1 active site contains both specific and promiscuous compartments, which enable the enzyme to act on structurally distinct chemicals. The position of bound homatropine, a cocaine analogue, and bound naloxone, a heroin analogue, reveal how hCE1 utilizes its large flexible pocket promiscuously and its small rigid pocket specifically. These crystal structures together with the results from atomic force microscopy provide the first evidence of hCE1's trimer-hexamer equilibrium.
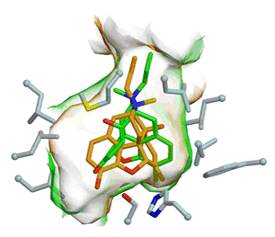
A selective surface ligand-binding site regulates the trimer-hexamer equilibrium and allows each monomer to bind cocaine and heroin analogs simultaneously.
Third, we have presented the structure of hCE1 in complexes with an anti-Alzheimer drug tacrine (Bencharit et al., 2003b). The manner in which tacrine binds, and the shape and size of hCE1 substrate binding gorge, are distinct from the tacrine bound acetylcholinesterase structure determined previously. Furthermore, tacrine appears in multiple conformations within hCE1's substrate binding gorge. We conclude that hCE1's ability to bind the same ligand in up to five conformations may contribute to its promiscuity and facilitate its ability to process variety of endogenous and exogenous compounds (Fig 3).
Diffraction data for the rCE, hCE1-homatropine, and hCE1-tacrine structures were collected at SSRL Beam Lines 7-1, 9-1, and 9-2. Diffraction data for the hCE1-naloxone structure was collected at the Advanced Photon Source (APS); SER-CAT Beam Line 22-ID.
- Redinbo MR, Bencharit S, Potter PM. Human carboxylesterase 1: from drug metabolism to drug discovery. Biochem. Soc. Trans. 2003;31:620-4.
- Bencharit S, Morton CL, Howard-Williams EL, Danks MK, Potter PM, Redinbo MR. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat. Struct. Biol. 2002;9:337-42.
- Bencharit S, Morton CL, Xue Y, Potter PM, Redinbo MR. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat. Struct. Biol. 2003a;10:349-56.
- Bencharit S, Morton CL, Hyatt JL, Kuhn P, Danks MK, Potter PM, Redinbo MR. Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine. From binding promiscuity to selective inhibition. Chem. Biol. 2003b;10:341-9.
S. Bencharit, C. L Morton, Y. Xue, P. M Potter and M. R Redinbo, "Structural Basis of Heroin and Cocaine Metabolism by a Promiscuous Human Drug-processing Enzyme", Nat. Struct. Biol. 10, 349 (2003)

