One goal of genome projects is to systematically identify genes (1,2). The best current knowledge indicates that humans carry approximately 35000 genes. This number is an estimate that varies from expert to expert and range up to 100,000 (3-5). To anyone who has taken an elementary biology class, this ambiguity must seem strange. How hard can it be to count genes? After all, don't cells translate genes into proteins? Counting genes should be as easy as counting proteins.
In fact, most estimates for the number of genes in the human genome exploit this strategy with researchers developing various ways of directly or indirectly counting proteins (6-10). The fundamental problem then is in the definition of a gene. A gene encodes an action - and not all the action in a cell is in the protein.
Watson and Crick proposed a central tenet of biology over 50 years ago (11): DNA ® RNA ®Protein. RNA thus functioned simply as a genetic intermediary between DNA and proteins. Even as this tenet was proposed, researchers knew that it was at best an approximation. If RNA served only as an intermediary, there should be some correspondence between the concentrations of amino acids of proteins and the nucleotides of RNA and DNA. Experimentally, this was not observed. Indeed, shortly after outlining how cells translated DNA to RNA to proteins, Crick observed "The evidence presented there showed that our ideas were in some important respects too simple" (12).
In 1983, Thomas Cech made a key discovery that led to his Nobel Prize. He discovered that an RNA molecule catalyzed a structural change in a molecule; in this case an RNA molecule (13). Until his discovery, catalysis was a role reserved for proteins. With Cech's discovery, the "action" happened in the RNA.
Knowledge of RNA's role within the cell has grown quickly. We now know that RNA plays a pivotal role in gene silencing, gene shuffling, protein regulation and even disease. As one example, researchers in Finland traced the cause of an inherited dwarfism to a specific location in the human genome (14). They sequenced the entire region only to find that people with and without the disease had exactly the same protein-encoding genes. They eventually traced the cause of disease to differences in the space between genes. These differences resulted in mutated 267 nucleotide non-coding RNA - RNA that did not result in protein production in either healthy or afflicted people - that triggered the onset of the disease.
Why did it take 30 years for someone to discover Crick's missing piece of the biological puzzle? The problem is entirely experimental. Many of biologists' most powerful tools do not work on RNA. For example, the Protein Data Bank currently contains about 20,000 protein structures. In contrast, the Data Bank contains the structures of only about 500 RNA molecules. Because of their charged phosphate backbones, RNA molecules resist crystallization and hence it is not possible to use x-ray crystallography to determine the 3-dimensional structure of RNAs. Similarly, circular dichroism - a structural tool prevalent in protein chemistry - yields little information when applied to RNA. Until recently, plight of the RNA experimentalist was quite bleak.
We have exploited small angle X-ray scattering (SAXS) to address this challenge (15-17). In many respects, our efforts represent an ideal marriage of a problem and technique. SAXS provides a quantitative measure of structure formation and conformational change, information typically not available in the field of RNA research. RNA, in turn, has properties that make it an excellent material for SAXS investigation. SAXS works by scattering off the difference in electron density between solvent (water) and the biological molecule. This "contrast" is relatively low for proteins, molecules composed primarily of C/N/O. RNA, with its electron-rich phosphate backbone, yields much better contrast (and data) at comparatively low concentrations. With polymerase chain reactions (PCR), designing systems to test an idea or take measurements is relatively straightforward. Finally, radiation induced aggregation - which can be a significant problem in SAXS experiments, appears largely lacking with RNA.
In our initial studies, we used the same catalytic RNA - generically termed a ribozyme - discovered by Cech (Figure 1).
The goal of our studies is simple: by understanding how this and other RNAs assume a 3-dimensional shape, we hope to develop rules that describe how a 1-dimensional chain of nucleotides spontaneously takes on a biologically active 3-dimensional shape. In protein studies and genomics, this is referred to as "solving the second genetic code", a problem of paramount importance in basic biophysics as well as having very practical applications like drug discovery.
As our initial question, we asked what drove the folding of this ribozyme. For example, collapse of the RNA coil might be driven by "dielectric screening". Concentrations of salt might mask the charges on the RNA phosphates and allow the RNA chain to compact. From biochemical work, we knew that the RNA enzyme only catalyzed reactions in the presences of Mg2+. Experimentally, equating shape formation with the rate of catalysis is problematic. The ribozyme might be folded or nearly folded without magnesium yet still not catalyze a reaction.
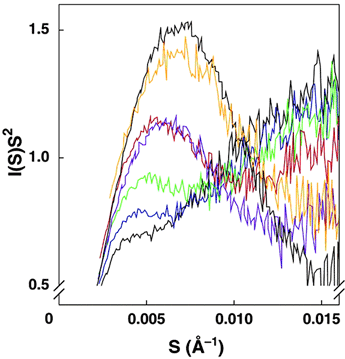
To test this, we looked at the structure of the ribozyme in varying concentrations of salts. We compared our measurements to the SAXS profile of the fully catalytic ribozyme, a structure achieved in low concentration of MgCl2. Our results, summarized in Figure 2, show that folding of the ribozyme is driven by divalent cations (catalysis is specific to Mg2+). Even if we account for charge differences, Mg2+ is at least 25 times more effective than Na+ in inducing structure in this ribozyme.
As RNA folds to its functional form, it must undergo compaction from a disordered chain to a specific structure. We used stopped-flow and continuous flow kinetics in conjunction with SAXS to directly monitor the compaction during folding of the Tetrahymena ribozyme over a time window of more than five orders of magnitude. We wanted to determine whether compaction occurs as an early step in folding, before specific tertiary structure formation, or whether it occurs as long-range contacts form, which by their nature necessitate collapse.
We observed substantial compaction in the low millisecond timescale, with overall compaction and global shape changes largely complete within one second. The earliest detected tertiary structure is formed at least 5-fold slower. Thus, compaction largely precedes specific tertiary structure formation, indicating that a nonspecifically collapsed intermediate is formed and transiently accumulates during folding (15). We must study other ribozymes to determine if this collapsed intermediate is a general feature in RNA structure formation.
We note that this "molten globule" or collapsed intermediate is not universally observed in proteins. When observed, protein molten globules are compact and almost entirely unstructured. The individual amino acids of the protein move about rapidly within the globule like disturbed bees in a hive. The helices and sheets that make up the structure of proteins are absent. In proteins, the creation of short-range secondary structure and the formation of long-range folded structure seem to coincide. In contrast, RNA secondary structure is formed in preparing the initial state (Figure 2) while final compaction is triggered by addition of magnesium.
If secondary structure is formed in the initial unfolded state, what impact does this structure have on folding? For example, one might simplistically argue that, since high concentrations seem to induce some secondary structure, the structure is already partially folded and would thus reach a folded "end-state" more quickly. Conversely, if the structure induced by salt was somehow incorrect, it might take more time for the ribozyme to assume the correct final shape.
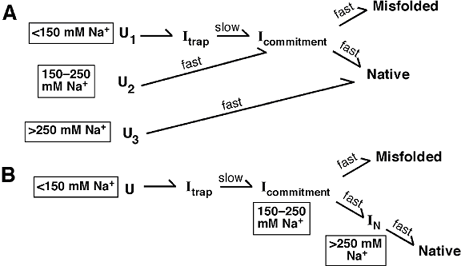
Two potential folding schemes, built from our and other authors' suggestions, are depicted in Figure 3. Scheme B follows from the simple suggestion above: increasing salt moves the ribozyme closer to the native folded form. Thus, at low salt, the ribozyme starts from an unfolded state. On addition of Mg2+, it moves to a long-lived intermediate termed Itrap, then to another intermediate Icommitment, and then either successfully folds or becomes trapped in an incorrect, misfolded structure. At higher salt concentrations, the ribozyme in Scheme B avoids the long-lived intermediate Itrap and folds more quickly.
Scheme A is much more complicated: the path the ribozyme takes when it folds depends on its starting state. At low salt, the pathway mimics that of Scheme B. At intermediate concentrations of Na+, the ribozyme's starting state is still unfolded but now has more secondary structure. On addition of magnesium, this path skips the trap species and moves to a final, potentially misfolded state. At still higher salt, the ribozyme folds directly to native species.
The folding of the ribosome follows Scheme A. The argument for this is in part based on the Kratky plots of Figure 2. We can prepare the long-lived intermediate Itrap. This intermediate is smaller than the unfolded ribozyme but larger than the misfolded or native final forms. We expect the species in Scheme B to have sizes intermediate between Itrap and the final states. Experimentally, this is not observed.
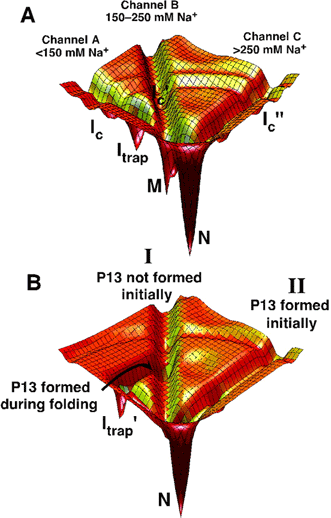
Structured RNAs achieve their active states by traversing complex, multidimensional energetic landscapes. By probing the folding landscape of the Tetrahymena ribozyme with single molecule fluorescence and SAXS, we have shown that the landscape contains discrete folding pathways. These pathways are separated by large free-energy barriers that prevent interconversion between them, indicating that the pathways lie in deep channels in the folding landscape (16) (Figure 4). In this, RNA structure formation differs drastically from that seen in proteins. In proteins, the starting state of unfolded protein has a comparatively small impact on the folding path and final folded form.
Taken together, these two studies have significant implications to our goal of transforming a 1-dimensional chain of RNA into a biologically relevant 3-dimensional shape. First, we have found that RNA forms a nonspecifically collapsed intermediate(s) and then searches for its tertiary contacts within a highly restricted subset of conformational space. This observation is a boon computationally. By forming "islands" or intermediates in the folding/search process, we can significantly speed the progress of our structural algorithms.
Unfortunately, we have also learned that RNA folding is highly path dependent. The observed intermediate and even the final folded product can depend on the initial conditions or, equivalently, the structure of the starting state. Ironically, correctly predicting the 3-dimensional structure of an RNA molecule may hinge on choosing the right shape for the unfolded "random coil".
This research was carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences.
- Collins, F. S. et al., "New goals for the US human genome project: 1998-2003." (1998)Science 282, 682-689.
- Eddy, S. R., "Non-Coding RNA Genes and the Modern RNA World." (2001) Nature Genet. 2, 919-929.
- Aparicio, S. A. J. R., "How to count ... human genes." (2000) Nature Genet. 25, 129-130.
- Hogenesch, J. B. et al., "A comparison of the Celera and Ensembl predicted gene sets reveals little overlap in novelgenes." (2001) Cell 106, 413-415.
- Wright, F. A. et al., "A draft annotation and overview of the human genome." (2001)Genome Biol. 2, 0025.1-0025.18.
- International Human Genome Sequencing Consortium. "Initial sequencing and analysis of the human genome." (2001) Nature 409, 860-921.
- Venter, J. C. et al., "The sequence of the human genome." (2001) Science 291, 1304-1351.
- Liang, F. et al., "Gene index analysis of the human genome estimates approximately 120,000 genes." (2000) Nature Genet. 25, 239-240.
- Ewing, B., Green, P., "Analysis of expressed sequence tags indicates 35,000 human genes." (2000) Nature Genet. 25, 232-234.
- Crollius, R. H. et al., "Estimate of human gene number provided by genome-wide analysis using Tetraodonnigroviridis DNA sequence." (2000) Nature Genet. 25, 235-238.
- Watson, J. D., Crick, F. H. C. (1953) Nature 171, 964-967.
- Crick, F. H. C. (1959) Brookhaven Symp. Biol. 12, 35-39.
- Cech, T. R., "Catalytic RNA: Structure and Mechanism." (1993) Biochem. Soc Trans. 21, 229-234.
- Ridanpää, M., Eenennaam, H. V., Pelin, K., Chadwick, R., Johnson, C., Yuan, B., Venrooij, W. V., Pruijn, G., Salmela, R., Rockas, S., Mäkitie, O., Kaitila, I., Chapelle, I., "Mutations in the RNA Component of RNase MRP Cause a Pleiotropic Human Disease, Cartilage-Hair Hypoplasia." (2001) Cell 104, 195-203.
- Russell, R., Millett, I. S., Tate, M. W., Kwok, L. W., Nakatani, B., Gruner, S. M., Mochrie, S. G. J., Pande, V., Doniach, S., Herschlag, D., Pollack, L., "Rapid Compaction During RNA Folding." (2002) Proc. Nat. Acad. Sci. 99(7), 4266-4271.
- Russell, R., Zhuang, X., Babcock, H. P., Millett, I. S., Doniach, S., Chu, S., Herschlag, D., "Exploring the Folding Landscape of a Structured RNA." (2002) Proc. Nat. Acad. Sci. 99(2), 155-160.
- ussell, R., Millett, I. S., Doniach, S., Herschlag, D., "Small Angle X-ray Scattering Reveals a Compact Intermediate in Folding of the Tetrahemena Group I RNA Enzyme." (2000) Nat. Struct. Biol. 7(5), 367-370.
R. Russell, I. S Millett, M. W Tate, L. W Kwok, B. Nakatani, S. M Gruner, S. G J Mochrie, V. Pande, S. Doniach and L. Pollack, "Rapid Compaction During RNA Folding", Proc. Natl. Acad. Sci. USA 99, 4266 (2002)
R. Russell, X. Zhuang, H. P Babcock, I. S Millett, S. Doniach, S. Chu and D. Herschlag, "Exploring the Folding Landscape of a Structured RNA ", Proc. Natl. Acad. Sci. USA 99, 155 (2002)




