
Sorption reactions on particle surfaces can dramatically affect the speciation, cycling and bioavailability of essential micronutrients (i.e. PO43-, Cu, Zn etc.) and toxic metals and metalloids (i.e. Pb, Hg, Se, As) in soils and aquatic environments. Considerable attention has been focused on understanding metal sorption reactions at a molecular/mechanistic level and the effects of metal concentration, pH, ionic strength, and complexing ligands on the ways in which metal ions bind to the surfaces of common mineral phases such as Fe-, Mn- and Al-(hydr)oxides and clays. However, a significant fraction of mineral surfaces in natural environments are extensively colonized by microbial organisms, which can also be potent sorbents for metals due to the large number of reactive functional groups that decorate the cell walls and outer membranes of bacterial surfaces.
Bacteria are widespread in soil and aquatic environments and are predominantly found in biofilm communities. Biofilms form when bacterial consortia attach to mineral surfaces and produce films of hydrated extracellular polymers. Once biofilms form, the bacterial-mineral micro-aggregates create complex interfaces with the surrounding aqueous solution. The biofilms may act as an "insulating layer" between the solution and the mineral surface or form "microenvironments" in which the local solution conditions are different than those in the bulk solution. Reactive functional groups, such as carboxyl, hydroxyl, amino and phosphoryl moieties, present on the bacterial surfaces and exopolysaccharide matrix, can provide a large array of binding sites for metals, and they may simultaneously block surface sites on the underlying substrate. In addition, bacterial activity may catalyze the transformation of toxic metals into less (or more) toxic species or enhance the dissolution of the underlying mineral substrate. All of these combined interactions may strongly affect the mechanisms and kinetics of metal sorption reactions in soils and aquatic environments.
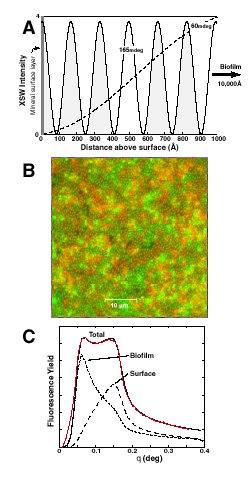
In Gordon Brown's group at Stanford University, new research has focused on the application of synchrotron-based x-ray spectroscopic techniques to the study of metal sorption reactions (e.g. Pb and Se) at biofilm-mineral-solution interfaces, similar to previous studies of metal sorption at biofilm-free mineral-solution interfaces. However, it is particularly challenging to investigate metal-complexation reactions at these more complex interfaces due to the variety of binding sites available for metals, the small length-scale of variation in the metal distributions (angstroms to nanometers) and the need to perform measurements under in situconditions. Recent work by Templeton et al. (PNAS 98, 11897 (2001)) has shown how the long-period x-ray standing-wave (XSW) technique can be used to probe metal-ion distributions within Burkholderia cepacia biofilms formed on Al- and Fe-oxide single-crystal surfaces. XSW fluorescence-yield profiles were collected for biofilms formed onα-Al2O3 and α-Fe2O3 surfaces that had been exposed to solutions with a range of Pb(II) concentrations. The data show that Pb(II) ions preferentially bind to highly reactive sites on the oxide surfaces. The order of Pb(II) reactivity with the mineral substrates followed the order α-Fe2O3 (0001)> α-Al2O3(1 02) > α-Al2O3 (0001), as expected from previous work in "clean" model systems without bacteria present. The combined data demonstrate that although the biofilm does form a large sink for Pb(II), especially at high Pb concentrations, the formation of the biofilm has not passivated the intrinsic reactivity of the underlying mineral surfaces.
A. S. Templeton, T. P. Trainor, S. J. Traina, A. M. Spormann and G. E. Brown, Jr., "Pb(II) Distributions at Biofilm-metal Oxide Interfaces ", Proc. Natl. Acad. Sci. USA 98, 11897 (2001)




