 The combined use of x-ray crystallography and solution small angle x-ray scattering has enabled a research collaboration involving scientists from Boston College and SSRL to provide structural evidence supporting a 30-year old model accounting for the cooperative binding of ligands to allosteric proteins and enzymes - a function central to physiology and cellular processes.
The combined use of x-ray crystallography and solution small angle x-ray scattering has enabled a research collaboration involving scientists from Boston College and SSRL to provide structural evidence supporting a 30-year old model accounting for the cooperative binding of ligands to allosteric proteins and enzymes - a function central to physiology and cellular processes.
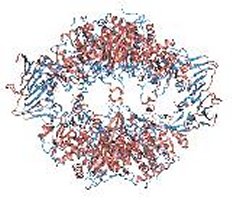
Over 30 years ago, two major models were developed to account for the cooperativity observed in oligomeric allosteric proteins such as hemoglobin, the oxygen carrier protein in blood: the concerted model, in which a protein has only two ”all-or-none” global states, vs. the sequential model that allows a number of different global conformational/energy states. Both, however, are based on just two local states of building blocks (subunits) in close analogy to magnetic spin states. In either model, a transition of one or more protein subunits leads to the global transition, in the case of hemoglobin, from the oxygen-releasing form to the oxygen-binding form, depending on the oxygen level in the blood stream. The concerted model, based on highly positive cooperativity, resembles the ferromagnetic phase transition, in which only two spin states account for the sharp phase transition between two global states. The sequential model, on the other hand, permits mixture of active and inactive subunits. Macol et al., constructed a version of an allosteric enzyme E. coli aspartate transcarbamoylase, which is composed of six equivalent catalytic monomers and six equivalent regulatory monomers in its native form, in such a way that only one of the six catalytic monomers could bind a substrate analog. Using solution x-ray scattering data recorded at BL4-2 to monitor the global structural state, they provided the first structural evidence that the transition of only one catalytic monomer is sufficient to transform the entire enzyme into the highly active state, lending strong support to the concerted model.
Funding Acknowledgement
This work was supported by the National Institute of General Medical Sciences. The Stanford Synchrotron Radiation Laboratory (SSRL) is operated by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Biology Resource is supported by the National Institutes of Health, National Center for Research Resources and by the Department of Energy, Office of Biological and Environmental Research.
Christine P. Macol, Hiro Tsuruta, Boguslaw Stec and Evan R. Kantrowitz, "Direct Structural Evidence for a Concerted Allosteric Transition in Escherichia coli Aspartate Transcarbamoylase", Nature Struct. Biol. 8, 423 (2001)




