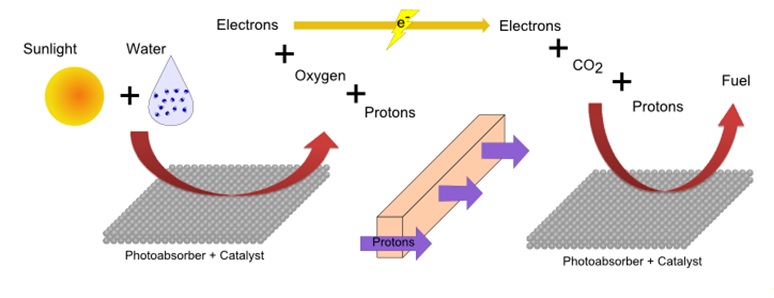
Plants and other organisms use a process called photosynthesis to produce carbohydrates and oxygen from water and carbon dioxide using sunlight. Artificial photosynthesis replicates this process to produce energy in the form of usable fuels for human needs. Researches have been developing devices for artificial photosynthesis, in which an assembly of catalysts and photoabsorbers can produce fuel from water, carbon dioxide and sunlight (Fig. 1). Due to its slow kinetics, the electrochemical oxidation of water, which splits water molecules into electrons, protons and oxygen molecules, is one of the particularly challenging aspects of this device. The use of effective catalysts has been essential in the development of artificial photosynthesis devices. Of the many materials, iridium oxide has been shown to have high reactivity and excellent stability and is unique as a catalyst under acidic conditions.
In a recent study, published in Angewandte Chemie International Edition, a research team from SSRL and SUNCAT at SLAC and Stanford University and the Joint Center for Artificial Photosynthesis (JCAP) at the Lawrence Berkeley National Laboratory probed the form of the iridium oxide catalyst during water oxidation. JCAP, a multi-institute research program and the largest U.S. Department of Energy (DOE) Energy Innovation Hub, is dedicated to the development of an artificial solar-fuel generation technology.
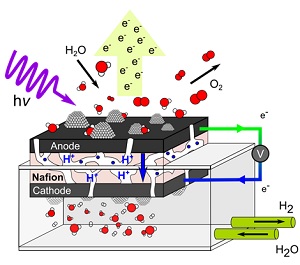
The team developed an electrochemical cell, shown in Fig. 2, that is compatible with the ambient-pressure x-ray photoelectron spectroscopy (XPS) end-station at SSRL’s Beam Line 13-2 [1,2]. With this setup, the team was able to follow the chemical changes of the catalyst in the course of the oxygen-generating water oxidation reaction. Through the observation of core-level shifts in x-ray photoelectron spectroscopy, the team identified a partial hydroxylation of iridium oxide upon exposure to water. The team also determined a change in the iridium oxidation state from +4 to +5 accompanied by a decrease in hydroxylation of iridium under water oxidation conditions. Moreover, the team probed different depths of the catalyst by tuning the x-ray photon energy and concluded that the observed changes associated with catalytic water oxidation are confined to the surface region of the catalyst.
These in situ findings support the idea that the water oxidation reaction on iridium oxide favors the single site mechanism through deprotonation of OOH over the dual site mechanism. This mechanistic understanding will provide the basis for the development of future cost-effective, alternative catalysts for artificial photosynthesis.
- S. Kaya, H. Ogasawara, L.-A. Näslund, J.-O. Forsell, H. Sanchez Casalongue, D. J. Miller and A. Nilsson, "Ambient-Pressure Photoelectron Spectroscopy for Heterogeneous Catalysis and Electrochemistry", Catal. Today 205, 101 (2013) doi: 10.1016/j.cattod.2012.08.005
- H. Sanchez Casalongue, S. Kaya, V. Viswanathan, D. J. Miller, D. Friebel, H. A. Hansen, J. K. Nørskov, A. Nilsson and H. Ogasawara, "Direct Observation of the Oxygenated Species during Oxygen Reduction on a Platinum Fuel Cell Cathode", Nat. Commun. 4, 2817 (2014) doi: 10.1038/ncomms3817
H. G. Sanchez Casalongue, M. Ling. Ng, S. Kaya, D. Friebel, H. Ogasawara and Nilsson, "In Situ Observation of Surface Species of Iridium Oxide Nanoparticles during the Oxygen Evolution Reaction", Angew. Chem., Int. Ed. Engl. 53, 7169 (2014) doi: 10.1002/ange.201402311




