Malaria is one of the most devastating parasitic diseases worldwide, amounting to 300 to 500 million cases and ~2 million deaths per year (1). Multiple species of Plasmodium infect the human host, the most important ones being P. falciparum and P. vivax. Increased occurrence of multi-drug resistant Plasmodium strains reflects the need for new effective antimalarials. Human infection starts when Anopheline mosquitoes inject sporozoites into the skin during a blood meal. The sporozoites gain access to the blood stream and invade liver cells where they develop and multiply. Upon rupture of the infected liver cell, merozoites are released and rapidly enter red blood cells, where they undergo schizogony (cell multiplication) and propagate the blood cycle of the infection that causes the malaria symptoms (2).
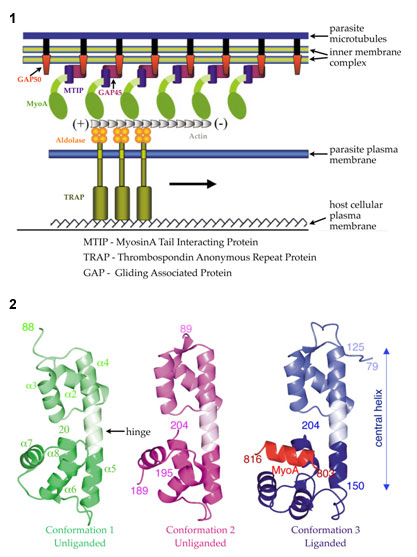
A multi-protein complex located in the narrow space between the parasite plasma membrane and the microtubule-supported inner membrane complex empowers both substrate-dependent gliding motility and host cell invasion in Plasmodium (3). This invasion machinery (Fig. 1) is highly conserved and required for leaving and entering different types of host cells. Blocking one or more interactions of the invasion machinery with small molecules inhibitors could provide effective novel antimalarials. The current study provides atomic level insights into a crucial protein interaction occurring in this essential multi-protein assembly of the malaria parasite.
Using crystallographic data collected at SSRL beamline 9-2 and at the ALS, Wim Hol's group have solved the structure of a complex of P. knowlesi Myosin A-tail interacting protein (MTIP) and MyoA-tail to 2.6 Å (P. knowlesi is a rodent parasite. P. falciparum and P. knowlesi share 71% sequence identity). The crystals belonged to space group P63 and contained 3 different conformations of MTIP in the asymmetric unit with one subunit bound with MyoA-tail (Fig. 2). The Myosin A-tail interacting protein bridges between the membrane associated proteins GAP45/GAP50 and the C-terminal tail of Myosin, which interacts with short actin filaments (Fig. 1). The actin filaments are attached to the glycolytic enzyme aldolase, which needs to be multimeric to connect actin and TRAP. TRAP recognizes specific host cell receptors on red blood cells initiating the process of invasion (Fig. 1).
A combination of hydrophilic and hydrophobic interactions is responsible for forming the complex between the tail and the tail-binding protein (Fig. 2). MyoA residues Gln-808 and His-810 make numerous hydrogen bonds with MTIP, whereas electrostatic interactions mainly involve the first and third residues from the conserved tribasic RKR motif spanning residues 812-814 in the MyoA-tail. MyoA Arg-812 forms a salt bridge with MTIP Glu-180, whereas MyoA Arg-814 is interacting with the side chain of MTIP Asp-202. Mutagenesis to Ala of each of the two MyoA-tail Arg residues of the tribasic motif, and each of the three hydrophobic MTIP-facing residues, results in a failure to interact with MTIP, which is in complete agreement with the structure of the MyoA-MTIP complex.
The viability as a drug target was tested by in vivo inhibition experiments of P. falciparum cultures using the MyoA-tail. The binding pocket for MyoA-tail provided by the C-terminal domain of MTIP displays significant differences to the human homolog. These differences can be exploited for structure based drug design of small molecules mimicking the hydrophobic side chains of MyoA-tail yielding a higher binding affinity and specificity.
This work was supported in part by NIH/NIGMS Grant 1P50 GM64655-01, Structural Genomics of Pathogenic Protozoa and NIH Grant AI48226.
- Snow, R. W., Guerra, C. A., Noor, A. M., Myint, H. Y., & Hay, S. I. (2005) Nature 434, 214-217.
- Baird, J. K. (2005) New England Journal of Medicine 352, 1565-1577.
- Bergman, L. W., Kaiser, K., Fujioka, H., Coppens, I., Daly, T. M., Fox, S., Matuschewski, K., Nussenzweig, V., & Kappe, S. H. I. (2003) J. Cell Sci. 116, 39-49.
Bosch, J., Turley, S., Daly, T. M., Bogh, S. M., Villasmil, M. L., Roach, C., Zhou, N., Morrisey, J. M., Vaidya, A. B., Bergman, L. W., et al. (2006). Structure of the MTIP-MyoA complex, a key component of the malaria parasite invasion motor. Proc. Natl. Acad. Sci. U. S. A. 103, 4852-4857




