Since the discovery that RNA can be an enzyme, a fundamental question has emerged: How does an RNA molecule fold up into a precise three-dimensional structure capable of catalyzing a chemical reaction? This problem is interesting not only from the point of view of living organisms, but also in terms of trying to understand how a pre-biotic "RNA World" populated by ribozymes, as evolutionary precursors of today's protein enzymes found in all living organisms, might have functioned.
The first ribozyme structure to be elucidated was that of the hammerhead RNA, a small self-cleaving RNA1,2. The hammerhead ribozyme has been the subject of intensive investigations as it is a small, tractable archetype for studying RNA catalysis. Two crystal structures containing a minimal RNA construct that supported the self-cleaving reaction were obtained in 1994 and 1995, and appeared to be in close agreement, yet were unable to explain a growing number of biochemical experimental results.
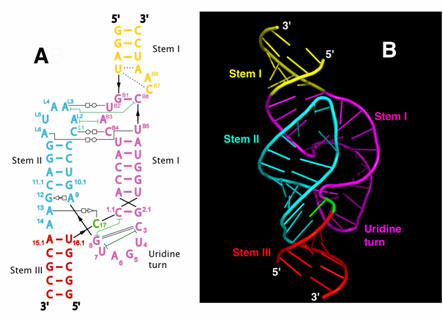
In 2003, a more extensive (or "full-length") hammerhead RNA sequence was discovered to be 1000-fold more catalytically active3,4. To understand the basis for this catalytic enhancement, and to address the accumulating concerns regarding the earlier structures, William G. Scott's group at UCSC solved the structure of a full-length hammerhead ribozyme using X-ray diffraction data collected at Beamline 1-5 at SSRL. These latest structural results suggest how the RNA itself, rather than functioning as passive scaffold for divalent metal ions, is a direct participant in its own self-cleavage chemistry. In the new structure, invariant residues appear positioned for general base and general acid catalysis in much the same way histidines are positioned for general acid-base catalysis in RNase A (Figure 2). Hence it appears these aspects of acid-base catalysis are so fundamental that they might be considered universal principles of macromolecular enzymology (both for proteins and RNAs).
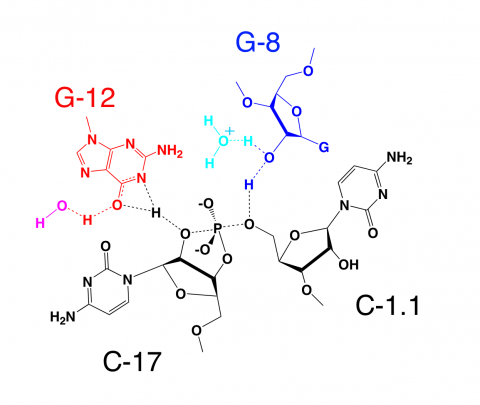
In addition to revealing the positions that several of the invariant nucleotides in the hammerhead sequence adopt in order to influence catalysis, the new hammerhead ribozyme structure reveals how an extensive tertiary contact forms between a hairpin loop capping Stem II and an unpaired region of Stem I. This contact, absent in the minimal hammerhead ribozyme structure, imparts a severe distortion upon Stem I, ultimately resulting in the distal end of Stem I becoming coaxial with Stems II and III. This tertiary contact forms much of the "top" half of the molecule as shown in Figure 1.
The new structural results help to explain a large set of discrepancies between the previous structural studies and numerous biochemical experiments, thanks to an apparent rearrangement of the active site (relative to the previous structures) that is consistent with the known biochemical constraints on the set of possible catalytic mechanisms. However, it is also clear from the new structure how cleavage could be observed within crystals of the minimal form of the hammerhead ribozyme.
Focusing upon the nucleotides that both structures have in common, it is apparent that a continuous deformation of the RNA can occur that transforms the previous structure into the newly observed, active structure. Using adiabatic morphing, one can compute an energetically plausible trajectory in which comparatively low-energy torsion-angle conformational changes transform the old structure into the new one in such a way that the overall fold and position of all three helical stems is approximately preserved, indicating a similar set of conformational changes may occur in solution or in crystals of the minimal hammerhead RNA. These are best viewed as a molecular movie (requires a QuickTime browser plugin).
This work was supported by the National Institutes of Health grant AI043393 and the Center for the Molecular Biology of RNA at University of California at Santa Cruz.
- Pley, H.W., Flaherty, K.M., and McKay, D.B. (1994). Three-dimensional structure of a hammerhead ribozyme. Nature 372, 68-74.
- Scott, W.G., Finch, J.T., and Klug, A. (1995). The crystal structure of an all-RNA hammerhead ribozyme: a proposed mechanism for RNA catalytic cleavage. Cell 81, 991-1002.
- Khvorova, A., Lescoute, A., Westhof, E., and Jayasena, S.D. (2003). Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity. Nat. Struct. Biol. 10, 708-712.
- De la Pena, M., Gago, S., and Flores, R. (2003). Peripheral regions of natural hammerhead ribozymes greatly increase their self-cleavage activity. EMBO J. 22, 5561-5570.
Monika Martick and William G. Scott, Tertiary Contacts Distant from the Active Site Prime a Ribozyme for Catalysis Cell 126: 309-320 (2006).




