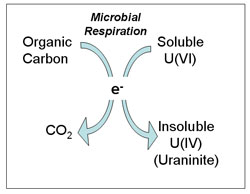
Uranium contamination of ground and surface waters has been detected at numerous sites throughout the world, including agricultural evaporation ponds (1), U.S. Department of Energy nuclear weapons manufacturing areas, and mine tailings sites (2). In oxygen-containing groundwater, uranium is generally found in the hexavalent oxidation state (3,4), which is a relatively soluble chemical form. As U(VI) is transported through groundwater, it can bond to surfaces of minerals, a process which may retard its transport (5-8). It has recently been shown, however, that U(VI) also bonds strongly to the common groundwater species carbonate and calcium to form stable dissolved ternary complexes, which can effectively compete with mineral surfaces as "reservoirs" for U(VI) (9). As a consequence, significant amounts of U(VI) remain in groundwater, thus maintaining relatively high mobilities for U(VI), a highly undesirable scenario. Conversely, the tetravalent oxidation state, U(IV), forms sparingly soluble solids, even in the presence of dissolved carbonate and calcium, and thus tends to be relatively immobile. Therefore, the oxidation state of uranium may play an important role in determining its environmental mobility (10). Numerous common, dissimilatory metal (DMRB) and sulfate reducing bacteria (SRB), including Shewanella, Geobacter, and Desulfovibrio species, couple the oxidation of organic matter and H2 to the reduction of U(VI), resulting in U(IV) and the subsequent precipitation of uraninite (UO2) (Figure 1) (11-13), a sparingly soluble phase. The idea of stimulating these biological processes for the purposes of stabilizing uranium in the subsurface is therefore promising as a basis for U remediation technologies, and has been investigated extensively at the beaker scale (14-16). While so-called bench-top measurements are valuable for quickly identifying promising research directions, soils and aquifers are too chemically and hydrologically complex to be realistically simulated in the laboratory. The long-term stability of biologically reduced uranium will be determined by the complex interplay of soil and sediment mineralogy, aqueous geochemistry, microbial activity, and potential U(IV) oxidants. Many of these factors have been studied under laboratory conditions; however, the impact of these factors on uranium cycling in natural, subsurface environments is still poorly understood. It is therefore essential to complement laboratory-based experiments with careful, long-term feasibility measurements of U(VI) reduction at contaminated field sites.

Our pilot-scale uranium bioremediation system located at the Y-12 facility (Area 3) at the Oak Ridge, TN, Field research center (FRC) provides a controlled subsurface environment in which these factors can be investigated. Over the course of 31 years, millions of gallons of plating wastes containing high concentrations of uranium and nitric acid were generated at this location and discharged into unlined ponds (the S-3 ponds) (Figure 2). In 1983, the ponds were capped and converted into a parking lot (Figure 2); however, uranium contamination remains and continues to migrate through subsurface fractures to surface discharge points. The U.S. Department of Energy (DOE) has established a Field Research Center (FRC) to assess the potential uranium immobilization through the stimulation of native populations of DMRB and SRB.
Since 2001, we have been performing uranium bioremediation experiments in FRC Area 3, immediately adjacent to the former S-3 Ponds (Figure 3). Prior to our remediation efforts, the uranium concentration (all in the hexavalent state) in groundwater at Area 3 was ~210 µM and the sediment contained up to 800 mg U kg-1 sediment, far in excess of the maximum allowable concentrations defined by the US EPA. A series of wells were installed at area 3 to control groundwater flow and allow the injection of solutes, such as ethanol, required to create a geochemical environment conducive for microbial growth and subsequent U(VI) reduction. The well system consisted of a nested recirculation system with a protective outer zone to isolate the inner remediation zone from the ambient geochemical conditions (Figure 3). Metal-reducing microbial activity in the inner zone was stimulated via ethanol addition, and dissolved uranium concentrations were monitored in the inner zone injection and extraction wells, and in a sampling well in the center of the bioremediation zone (Figure 3). No other such long-term field-scale research project has been undertaken to evaluate the efficacy of reductive bioremediation of U(VI) and evaluate how it could be scaled up to treat large contaminated sites.
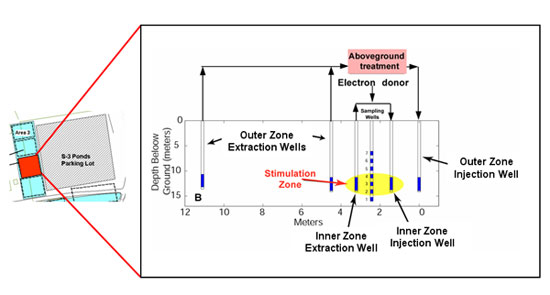
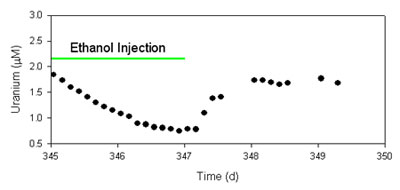
After several months of subsurface conditioning to created a low nitrate, neutral pH remediation zone (14), microbial uranium reduction was stimulated by injection of ethanol (1.0 - 1.5 mM) through the inner zone injection well (Figure 3). During the initial uranium remediation period (185 - 535 d of field site operation), dissolved uranium concentrations in the inner treatment zone decreased rapidly from 2 µM to <1 µM in response to ethanol addition, exemplified by Figure 4. Ethanol injection was repeated more than 50 times during this time period and resulted in similar trends in dissolved uranium concentration.
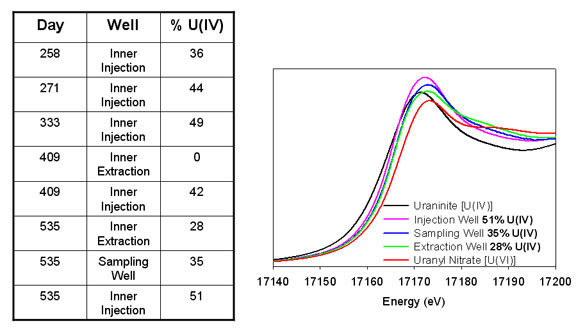
Although bacterial reduction of mobile U(VI) to immobile U(IV) is likely the mechanism responsible for the decrease in dissolved uranium concentration during ethanol addition, it is critical to confirm bacterial uranium reduction by measuring uranium's oxidation state in sediment samples. Sediment samples from the inner treatment zone wells and a sampling well were analyzed using X-ray absorption near edge structure (XANES) spectroscopy at SSRL Beam Line 11-2. Uranium oxidation state in the sediment samples was determined by comparison of the U LIII-edge XANES spectra of sediment samples to U(VI) and U(IV) standards (Figure 5) (17). Prior to biostimulation, U(IV) was not detectable in the subsurface of Area 3. Sediment samples were retrieved several times during the initial bioremediation period (Table 1). Partial reduction of U(IV) was first observed in the inner zone injection well on day 258, and U(IV) continued to accumulate in this well for the duration of the experiment (Table 1) Initially, U(IV) was not observed in the inner zone extraction well; however, by day 535, U(IV) was present throughout the bioremediation zone (Table 1, Figure 5).
Microbial activity has produced low dissolved uranium concentrations and high proportions of solid-phase U(IV) throughout the subsurface system remediation system. Continued biostimulation has resulted in groundwater uranium concentrations below the U.S. Environmental Protection Agency's drinking water standards (0.126 µM). Current research is examining the long-term stability of biologically reduced uranium, particularly in the presence of potential oxidants, including molecular oxygen (18). The long-term goal of this project is to decrease the flux of uranium leaving the site to the point that it is harmless.
This work was supported by the US DOE, Office of Biological and Environmental Remediation Program (ERSP) under grant DOEAC05-00OR22725. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. DOE, Office of Basic Energy Sciences. Environmental remediation sciences research at SSRL is supported by the ERSP support program.
- Bradford, G. R.; Bakhtar, D.; Westcot, D. Uranium, vanadium, and molybdenum in saline waters of California. Journ. Environ. Qual. 1990, 19, 105-108.
- Riley, R. G.; Zachara, J. M.; Wobber, F. J. "Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research," U.S. Department of Energy, 1992.
- Langmuir, D. Uranium solution-mineral equilibria at low temperature with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547-569.
- Sandino, A.; Bruno, J. The solubility of (UO2)3(PO4)2·4H2O(s) and the formation of U(VI) phospate complexes: Their influence in uranium speciation in natural waters. Geochim. Cosmochim. Acta 1992, 56, 4135-4145.
- Moyes, L. N.; Parkman, R. H.; Charnock, J. M.; Vaughan, D. J.; Livens, F. R.; Hughes, C. R.; Braithwaite, A. Uranium uptake from aqueous solution by interaction with goethite, lepidocrocite, muscovite, and mackinawite: An x-ray absorption spectroscopy study. Environ. Sci. Technol. 2000, 34, 1062-1068.
- Bostick, B. B.; Fendorf, S.; Barnett, M. O.; Jardine, P. M.; Brooks, S. C. Uranyl surface complexes formed on subsurface media from DOE facilities. Soil Sci. Soc. Am. J. 2002, 66, 99-108.
- Barnett, M. O.; Jardine, P. M.; Brooks, S. C.; Selim, H. M. Adsorption and transport of uranium(VI) in subsurface media. Soil Sci. Soc. Am. J. 2000, 64, 908-917.
- Barnes, C. E.; Cochran, J. K. Uranium geochemistry in esturaine sediments: Controls on removal and release processes. Geochim. Cosmochim. Acta 1993, 57, 555-569.
- Grenthe, I.; Fuger, J.; Konings, R. J. M.; Lemire, R. J.; Muller, A. B.; Nguyen-Trung, C.; Wanner, H. Chemical thermodynamics of uranium; North-Holland Elsevier Science Publishers B.V.: Amsterdam, 1992; Vol. 1.
- Liger, E.; Charlet, L.; Cappellen, P. V. Surface catalysis of uranium(VI) reduction by iron(II). Geochim. Cosmochim. Acta 1999, 63, 2939-2955.
- Fredrickson, J. K.; Zachara, J. M.; Kennedy, D. W.; Duff, M. C.; Gorby, Y. A.; Li, S. M. W.; Krupka, K. M. Reduction of U(VI) in goethite (a-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim. Cosmochim. Acta 2000, 64, 3085-3098.
- Lovely, D. R.; Phillips, E. J. P. Bioremediation of uranium contamination with enzymatic uranium reduction. Environ. Sci. Technol. 1992, 26, 2228-2234.
- Gorby, Y. A.; Lovley, D. R. Enzymatic uranium precipitation. Environ. Sci. Technol. 1992, 26, 205-207.
- Wu, W.-M.; Carley, J.; Fienen, M.; Mehlhorn, T.; Lowe, K.; Nyman, J.; Luo, J.; Gentile, M.; Rajan, R.; Wagner, D.; Hickey, R.; Gu, B.; Watson, D. B.; Cirpka, O.; Kitanidis, P.; Jardine, P. M.; Criddle, C. Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 1. Conditioning of a treatment zone. Environ. Sci. Technol. 2006, 40, 3978-3985.
- Wu, W.-M.; Carley, J.; Gentry, T.; Ginder-Vogel, M.; Fienen, M.; Mehlhorn, T.; Yan, H.; Caroll, S.; Pace, M.; Nyman, J.; Luo, J.; Gentile, M.; Fields, M. W.; Hickey, R.; Watson, D. B.; Cirpka, O.; Zhou, J.; Fendorf, S.; Kitanidis, P.; Jardine, P. M.; Criddle, C. Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 2. Geochemical control of U(VI) bioavailability and evidence of U(VI) reduction. Environ. Sci. Technol. 2006, 40, 3986-3995.
- Anderson, R. T.; Vrionis, H. A.; Ortiz-Bernard, I.; Resch, C. T.; Long, P. E.; Dayvault, R.; Karp, K.; Marutzky, S.; Metzler, D. R.; Peacock, A. D.; White, D. C.; Lowe, M.; Lovley, D. R. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microb. 2003, 69, 5884-5891.
- Bertsch, P. M.; Hunter, D. B. In situ chemical speciation of uranium in soils and sediments by micro x-ray absorption spectroscopy. Environ. Sci. Technol. 1994, 28, 980-984.
- Wu, W.-M.; Carley, J.; Luo, J.; Ginder-Vogel, M.; Cardanans, E.; Leigh, M. B.; Hwang, C.; Kelly, S. D.; Ruan, C.; Wu, L.; Gentry, T.; Lowe, K.; Mehlhorn, T.; Carroll, S. L.; Fields, M. W.; Gu, B.; Watson, D.; Kemner, K. M.; Marsh, T. L.; Tiedje, J. M.; Zhou, J.; Fendorf, S.; Kitanidis, P.; Jardine, P. M.; Criddle, C. In situ bioreduction of uranium(VI) to submicromolar levels and reoxidation by dissolved oxygen. Environ. Sci. Technol. 2006, Submitted.
W.-M. Wu, W.-M.; Carley, J.; Gentry, T.; Ginder-Vogel, M. A.; M. Fienen, M.; Mehlhorn, T.; Yan, H.; Carroll, S.; Pace, M. N.; Nyman, J.; Luo, J.; Gentile, M. E.; Fields, M. W.; Hickey, R. F.; Gu, B.; Watson, D.; Cirpka, O. A.; Zhou, J.; Fendorf, S.; Kitanidis, P. K.; Jardine, P. M.; Criddle, C. S. "Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 2. Reduction of U (VI) and geochemical control of U(VI) bioavailability", Environ. Sci. Technol. 2006, 40, 3986.




