The glial cell line-derived neurotrophic factor (GDNF), neurturin (NRTN), artemin (ARTN), and persephin (PSPN) are GDNF family ligands (GFLs) that are crucial for the development and maintenance of many neurons [1,2]. The trophic effect of GFLs on the dopamine and motor neurons has stimulated interest in their use for the treatment of neurodegenerative diseases such as Parkinson's. These structurally related neurotrophic factors signal by forming a ternary complex with a nonsignaling, ligand-specific GFRa receptor and a signaling and shared receptor tyrosine kinase RET. Four different GFRa receptors (GFRa1-4) have been identified. The preferential interactions between GFLs and GFRa receptors have also been established as GDNF to GFRa1, NRTN to GFRa2, ARTN to GFRa3, and PSPN to GFRa4 [3]. Given the importance of GFLs in basic neurobiology and their potential therapeutic value, it is a compelling goal to understand the molecular basis of the interactions between GFLs and their receptors.
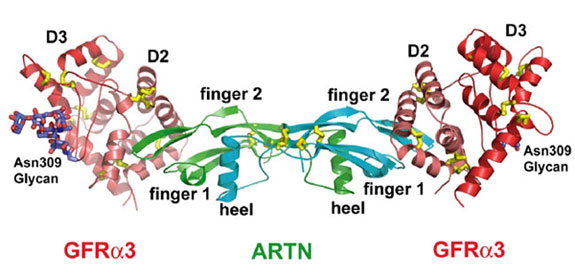
The structures of ARTN-GFRa3 binary complex and unbound ARTN in two crystal forms have been determined by a combination of heavy atom and molecular replacement methods using data collected at SSRL Beam Line 11-1 and at the ALS. The binary complex is composed of one ARTN homodimer and two truncated GFRa3 receptors consisting of the D2 and D3 domains (Figure 1). Instead of being two independent domains as people previously thought, the D2 and D3 domains were packed together to form a globular structure. Both D2 and D3 domains are folded as a triangle spiral, having disulfide bonds in the corners of the triangle to fix the fold. The ARTN monomer structure has two b sheet fingers, a cysteine-knot core motif, and an a-helical heel. Two ARTN monomers form a symmetric homodimer with an inter-chain disulfide bond.
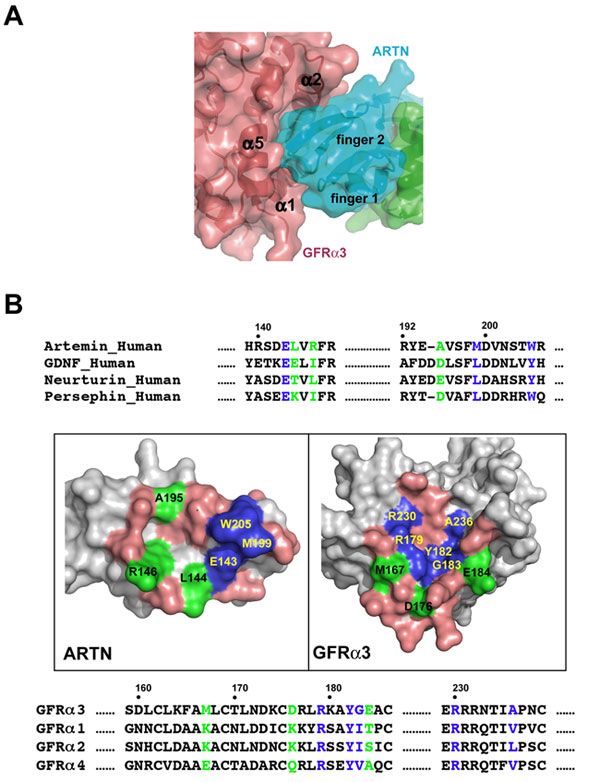
The complex structure of ARTN with GFRa3 revealed a convergent recognition mode for all GFLs. In the ARTN/GFRa3 binding interface, the tip ends of fingers 1 and 2 of ARTN insert into a pocket in the center of GFR 3 D2 domain surrounded by helices a1, a2, and a5 (Figure 2a). The ARTN/GFRa3 interface has two contact patches in the center, one hydrophobic and one hydrophilic, which are conserved in all GFL-GFRa pairs. The hydrophobic patch is composed of residues Met-199 and Trp-205 of ARTN and Tyr-182, Gly-183, and Ala-236 of GFRa3. All these positions are conserved as hydrophobic residues in other GFLs and GFRa receptors (Figure 2b). Residues Glu-143 of ARTN and Arg-179, Arg-230 of GFRa3 in the hydrophilic patch are strictly conserved in all GFL-GFRa pairs (Figure 2b). Mutations of the conserved positions in GDNF resulted in a completed loss of its binding activity for GFRa1 receptor [4]. While these residues clearly serve as common anchor points, the surrounding non-conserved residues may be responsible for the binding specificity between GFLs and GFRa receptors.
Based on the complex structure and other information, we have proposed two composite RET binding surfaces on the ARTN-GFRa3 binary complex, which would facilitate the recruitment of two RET receptors, leading to the close proximity of RET intracellular tyrosine kinase domains required for the signaling.
This work was supported by NIH grant RO1 HL077325, The Keck Foundation, The Christopher Reeve Paralysis Foundation, and The Howard Hughes Medical Institute.
- Airaksinen, M. S., and Saarma, M. (2002). The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3, 383-394.
- Baloh, R. H., Enomoto, H., Johnson, E. M., Jr., and Milbrandt, J. (2000). The GDNF family ligands and receptors - implications for neural development. Curr Opin Neurobiol 10, 103-110.
- Airaksinen, M. S., Titievsky, A., and Saarma, M. (1999). GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci 13, 313-325.
- Eketjall, S., Fainzilber, M., Murray-Rust, J., and Ibanez, C. F. (1999). Distinct structural elements in GDNF mediate binding to GFRa1 and activation of the GFRa1-c-Ret receptor complex. Embo J 18, 5901-5910.
Wang, X., Baloh, R.H., Milbrandt, J., and Garcia, K.C. (2006). Structure of artemin complexed with its receptor GFRa3: Convergent recognition of glial cell line-derived neurotrophic factors. Structure 14, 1083-1092.




