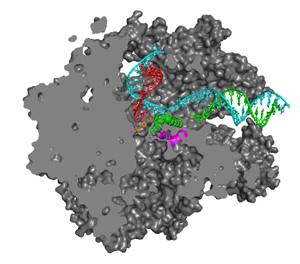
Transcription is the first step and the key control point in the pathway of gene expression. Transcriptional regulation underlies development, oncogenesis, and other fundamental processes. The central enzyme in transcription is RNA polymerase, in eukaryotic cells there are three forms of RNA polymerase, designated I, II, and III (or A, B, and C), made up of 10-15 polypeptides. The fundamental mechanism of transcription is conserved among cellular RNA polymerases. Common features include an unwound region, or "transcription bubble," of about 15 base pairs of the DNA template and some eight residues of the RNA transcript hybridized with the DNA in the center of the bubble. These enzymes are capable of both forward and retrograde movement ("backtracking") on the DNA. Forward movement is favored by the binding of nucleoside triphosphates (NTPs), while backtracking occurs especially when the enzyme encounters an impediment, such as damaged DNA. All NTP's can bind the entry or "E" site, whereas only an NTP matched for base pairing with the DNA template binds the A (addition) site for addition to the growing RNA chain (1).
The way in which the correctly matched and positioned NTP is recognized and how this recognition leads to catalysis remain obscure. The energies of base pairing and stacking are insufficient for base selectivity, and the question arises of why transient occupation of the A site by either incorrect NTP or 2'-dNTP substrates does not lead to erroneous RNA synthesis. Genetic and biochemical studies have implicated two conserved polymerase domains, termed F and G, in the transcription mechanism (2,3). Structural studies have identified these two domains with elements adjacent to the polymerase active site, termed the bridge helix (F) and trigger loop (G) (4). In the x-ray structures of transcribing complexes, however, no contact of these structural elements with NTP in the A or E sites has been observed. The present work describes a series of pol II transcribing complex structures that reveal such contacts and suggest the roles of these domains in the transcription mechanism (Figure 1).
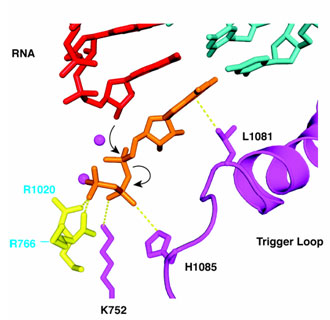
The trigger loop is a mobile element, allowing entry of NTP into the E and A sites in conformations previously observed, and sealing off the A site in the conformation reported here. Located beneath NTP in the A site, the trigger loop directly contacts the base and b-phosphate, and indirectly contacts 2'- and 3'-OH groups of the ribose sugar as well. Numerous interactions with other pol II residues serve to configure and position the trigger loop, so it reads out not only the chemical nature of the NTP but also the parameters of the DNA-RNA hybrid helix in the A site. A well-defined conformation of the trigger loop may be capable of readout to Ångstrom precision. Inasmuch as the hybrid helix differs substantially from B-form DNA (difference of 3 Å in minor groove width and 5.5 Å in root-mean- square phosphorous positions), such readout would readily distinguish ribo from deoxyribo NTPs, as well as providing powerful discrimination against purine-purine and pyrimidine-pyrimidine mispairing.
Two further features of trigger loop interaction may be crucial for transcription. First, the contact of His1085 with the NTP b-phosphate noted above may be key to catalysis. The distance between the imidazole N-H group and b phosphate oxygen is about 3.5 Å, optimal for hydrogen bonding or salt bridge interaction. The protonated imidazole group would be expected to withdraw electron density from the phosphate and facilitate SN2 attack of the RNA 3'- terminal OH group, leading to phosphodiester bond formation (Figure 2). Second, trigger loop interaction with NTP in the A site is evidently poised on the verge of stability, since the interaction could only be detected with improved data quality and analysis. If any feature of the NTP or its location is incorrect, the interaction will be lost.
The trigger loop may therefore couple nucleotide recognition to catalysis. In the presence of matched rNTP in the A site, it will swing into position and literally "trigger" phosphodiester bond formation (Figure 2). An incorrect NTP in the A site will not support trigger loop interaction and so is unlikely to undergo catalysis. When reaction with a correct NTP does occur, the release of pyrophosphate disrupts contact with His1085, likely destabilizing trigger loop interaction and freeing the DNA-RNA hybrid for translocation. Movement of the trigger loop, coupled to that of the bridge helix (Figure 2), may contribute to the translocation process (3,5).
- Westover, K. D., Bushnell, D. A., and Kornberg, R. D. (2004). Structural basis of transcription: nucleotide selection by rotation in the RNA polymerase II active center. Cell 119, 481-489.
- Allison, L. A., Moyle, M., Shales, M., and Ingles, C. J. (1985). Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell 42, 599-610.
- Bar-Nahum, G., Epshtein, V., Ruckenstein, A. E., Rafikov, R., Mustaev, A., and Nudler, E. (2005). A ratchet mechanism of transcription elongation and its control. Cell 120, 183-193.
- Cramer, P., Bushnell, D. A., and Kornberg, R. D. (2001). Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292, 1863-1876.
- Gnatt, A. L., Cramer, P., Fu, J., Bushnell, D. A., and Kornberg, R. D. (2001). Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 Å resolution. Science 292, 1876-1882.
Wang, D., Bushnell, D.A., Westover, K.D., Kaplan, C.D., and Kornberg, R.D. (2006) Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell 127, 941.




