Bacterial oxidation of Mn(II) impacts the global geochemical cycling of carbon, nitrogen, sulfur, nutrients, and contaminants in the environment. Manganese is abundant in the biosphere (~1014 Kg of suspended and dissolved manganese in the oceans) and is second only to iron in relative terrestrial abundance of transition metals. Manganese is an important nutrient in the marine water column and is fundamentally required for photosynthesis. The acquisition of manganese by organisms and the biogeochemistry of manganese in the oceans is therefore an essential part of global carbon fixation processes (i.e., uptake and conversion of CO2 to organic molecules, biomass). Manganese oxides are formed in sea water via bacterial catalysis of the oxidation of dissolved Mn(II) to Mn(IV) (Tebo et al., 2004). The oxides form external to bacterial cells (on cell surfaces or bacterial exopolymers) and subsequently settle through the marine water column, where they are available to react with dissolved ions. Bacteriogenic manganese oxides have high surface areas, are among the strongest sorbents of heavy metals, and are powerful oxidants of organic materials. Via sorption and oxidation/reduction reactions, settling manganese oxides help to mediate the trace element and nutrient composition of sea water. One of the most fundamental of scientific questions relating to this subject is, "What are the structures and compositions (i.e., the identities) of marine bacteriogenic manganese oxides?". Identifying marine manganese oxides will substantially enhance our ability to model and understand their roles in maintaining the chemistry of the oceans. This information will also directly contribute to a greater understanding of the properties of bacteriogenic manganese oxides, which are of great interest for their potential technological applications.
A collaborative group of scientists from SSRL and the Oregon Health and Science University have used the in-situ synchrotron-based techniques, EXAFS (extended x-ray absorption fine structure) spectroscopy and XRD (x-ray diffraction), to determine the identities of manganese oxides formed in sea water by the marine bacterium, Bacillus sp., strain SG-1. Both techniques provide information regarding the molecular-scale structures (i.e., the arrangement of atoms) of the bacteriogenic oxides. These techniques are highly complementary; EXAFS is primarily sensitive to variations in the local structure (up to about 6 Å) around manganese atoms and can be measured from noncrystalline substances such as amorphous colloids and species dissolved in solution. In comparison, XRD is highly sensitive to the long-range structure (up to about 20 Å), disorder, and particle size in crystalline materials averaged over the entire unit cell (i.e., the fundamental crystal structure repeat unit). Both techniques can be used to measure wet, undisturbed samples, which is critical for capturing the chemistry that occurs under natural wet conditions.
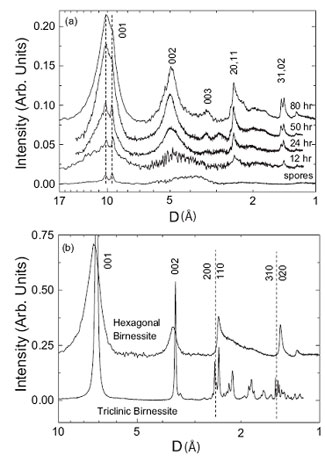
X-ray diffraction data for bacteriogenic manganese oxides grown in sea water are shown in Figure 2(a) (Webb et al., 2005a). The data for the samples reacted for ≥ 24 hr are similar to the x-ray diffraction pattern for the layered Mn(IV) oxide, c-disordered hexagonal birnessite (figure 2(b)); Most importantly, they exhibit strongly asymmetric (200)/(110) peaks (at ca 1.4 Å) and (310)/(020) peaks (ca 2.4 Å). This result qualitatively suggests that the bacteriogenic oxides are layered manganese oxides. The layer repeat distance of the bacteriogenic oxides is 10 Å, as indicated by the position of the (001) peak. Close inspection of the sample patterns reveals small peaks that indicate the presence of a related layered manganese oxide, triclinic birnessite. In particular, the presence of two (310)/(020) diffraction peaks and a small peak on the left-hand side of the (200)/(110) peak indicate that the birnessite layer unit cell has undergone a slight transformation (a slight lengthening along one of the unit cell axes), causing a breakdown of the initial hexagonal symmetry (the designation "triclinic" indicates the lowest possible unit cell symmetry). The x-ray diffraction pattern for the 12 hr sample shows only hexagonal birnessite peaks, suggesting that it is the initial phase. Note that the peaks are very weak and broad for this sample, indicating very poor crystallinity of the material. If bacteriogenic manganese oxides are grown in a NaCl solution, which is devoid of Ca2+, Mg2+ and other important ions in sea water, then the resulting primary bacteriogenic oxides remain very poorly crystalline (no diffraction peaks), and hexagonal birnessite is not produced over this time scale (Bargar et al., 2005; Webb et al., 2005b). This comparison suggests that one of the major ions in sea water is required to generate or stabilize these marine birnessites. Subsequent measurements have shown this ion to be Ca2+, which is incorporated in the interlayer of the oxide structure (Webb et al., 2005a).
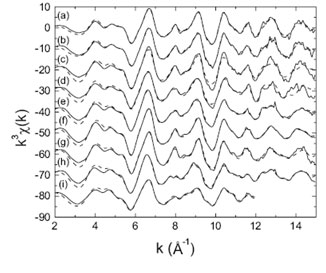
EXAFS spectra for bacteriogenic manganese oxides grown in sea water are shown in Figure 3. Visual inspection of figure 3 shows that the sample spectra ((a) - (e)) match the spectra for hexagonal birnessite (spectrum (g)) and s-MnO2 (spectrum (f)), which is a disordered form of hexagonal birnessite. Fits to the EXAFS spectra indicate the initial bacteriogenic product to be a layered hexagonal birnessite and provide the positions of atoms within the manganese oxide layer. EXAFS fits further indicate that the structure contains numerous Mn(IV) vacancy defects (~ 14% of all manganese sites are empty), which contribute substantially to the chemical reactivity of this material. With time, a small fraction of triclinic birnessite appears. Integration of the EXAFS and XRD results allows the structures of the bacteriogenic oxides to be reconstructed, as shown in figure 4.
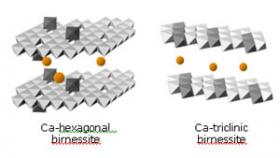
The conclusion from both sets of measurements is that the bacteriogenic manganese oxide produced in sea water is a poorly crystalline layered manganese oxide, birnessite. No other forms of manganese oxide were observed. One important process that this result helps to illuminate is the photocatalyzed reductive dissolution of manganese oxides in sea water (i.e., sunlight-driven reduction of Mn(IV) to Mn(II), followed by release of Mn(II)). This reaction occurs in the surface mixed layers of the oceans, where it helps to maintain a pronounced manganese concentration maximum (Sunda and Huntsman, 1988). Birnessite is particularly susceptible to photo-stimulated reductive dissolution (Sherman, 2005). Thus, the dominance of birnessite as the primary bacteriogenic marine manganese oxide would help to explain this chemical behavior in the surface layers of the oceans.
This work was support by the National Science Foundation, Chemistry Division and Earth Sciences Division. This research was carried out at the Stanford Synchrotron Radiation Laboratory, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
- Bargar J. R., Tebo B. M., Bergamann U., Webb S. M., Glaetzel P., Chiu V. Q., and Villalobos M. (2005) Biotic and abiotic products of Mn(II) oxidation by spores of the marine bacillus sp. strain SG-1. American Mineralogist 90, 143-154.
- Sherman D. M. (2005) Electronic structures of iron(III) and manganese(IV) (hydr)oxide minerals: Thermodynamics of photochemical reductive dissolution in aquatic environments. Geochimica et Cosmochimica Acta 69(13), 3249-3255.
- Sunda W. G. and Huntsman S. A. (1988) Effect of sunlight on redox cycles of manganese in the southwestern Sargasso Sea. Deep-Sea Research 35, 1297-1317.
- Tebo B. M., Bargar J. R., Clement B., Dick G., Murray K. J., Parker D., Verity R., and Webb S. (2004) Manganese biooxides: properties and mechanisms of formation. Annual Review of Earth and Planetary Science 32, 287-328.
- Webb S. M., Tebo B. M., and Bargar J. R. (2005a) Structural characterization of biogenic manganese oxides produced in sea water by the marine bacillus sp., strain SG-1. American Mineralogist 90, 1342 - 1357.
- Webb S. M., Tebo B. M., and Bargar J. R. (2005b) Structural influences of sodium and calcium ions on biogenic manganese oxides produced by the marine bacillus sp., strain SG-1. Geomicrobiology Journal 22, 181-193.
S. M. Webb, B. M. Tebo, and J. R. Bargar, "Structural Characterization of Biogenic Mn Oxides Produced in Seawater by the Marine Bacillus sp. Strain SG-1", Am. Mineral. 90, 1342 (2005)




