Nanometer-size metal particles are of fundamental interest for their chemical and quantum electronic properties, and of practical interest for many potential applications [1, 2]. Historically gold nanoparticles are the best studied, dating back to ancient Rome where colloidal gold was thought to have medicinal properties due to its blood red color. Gold has proven to have applications in medicine with some modern arthritis drugs using gold compounds. With all this interest in gold particles the structure of a thiol-protected gold nanoparticle had yet to be unambiguously determined. Electron microscopy
[3,4], powder x-ray diffraction [5] and theoretical studies [6] had led to the idea that gold nanoparticles would adopt closed geometric shells with crystalline packing. This would lead to defined core sizes such that the gold clusters would have a discrete number of atoms representing closed geometric shells [7]. For example icosahedrally packed gold clusters were predicated to have "magic number" sizes corresponding to 55, 147, 309. An alternative to the closed geometric model was the jellium model, which predicted closed electronic shells instead of geometric shells (reviewed in Rev. Mod. Phys 65, 611-676). Testing of these theories requires the unambiguous structural determination of a series of gold nanoparticles.
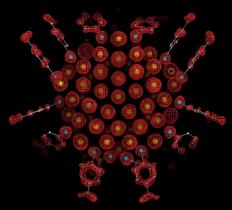
Structural determination of thiol protected gold nanoparticles has been complicated by the problem that particles are typically heterogenous as synthesized. Through systematic variation of solution conditions for gold nanoparticle synthesis, the Kornberg lab obtained particles sufficiently uniform in size for the growth of large single crystals opened the way to X-ray structure determination. SSRL Beam Lines 11-1 and 11-3 were utilized to perform X-ray analysis of these crystals resulting in the first unambigous determination of a thiol protected gold nanoparticle. The resulting electron density map revealed a particle of 102 gold atoms and 44 p-mercaptobenzoic acids (p-MBAs) (Figure 1). The structure was refined at a resolution of 1.15 Å to Rwork and Rfree of 8.8% and 9.5%.
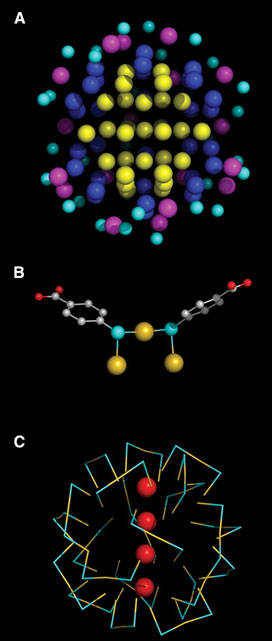
The Au102(p-MBA)44 structure revealed a metallic gold nucleus of 79 atoms packed with decahedral symmetry protected by a Au23(p-MBA)44 layer of gold-thiol oligomers. Gold atoms up to 5.5 Å from the center of the particle do not contact sulfur, those in a shell of radius 6.0 to 6.3 Å bind one sulfur, and those in a shell of radius 7.5 to 8.0 Å bind two sulfurs (Fig. 2A). All sulfur atoms lie in a shell of radius 8.3¬ ± 0.4 Å and bind in a bridge conformation [8] to two gold atoms, at least one of which binds two sulfurs, forming a "staple" motif (Fig. 2B, C). The gold-sulfur distance ranges from 2.2 to 2.6 Å. Gold-sulfur-gold angles are 80 to 115° and sulfur-gold-sulfur angles are 155 to 175°. If the surface is taken as all gold atoms interacting with sulfur, then the coverage by p-MBA (thiol:gold ratio) is 70%, much higher than the values of 31% and 33% for benzenethiol [9] and alkanethiols [10] on Au(111) surfaces, reflecting the curvature of the nanoparticle surface.
The very existence of a discrete Au102(p-MBA)44 nanoparticle is a notable finding from this work. Discrete sizes have been explained in the past by geometrical or electronic shell closing. The arrangement of gold atoms, with polar caps and an equatorial band, argues against geometrical shell closing. If, however, each gold atom (5d10 6s1) contributes one valence electron, and 44 are engaged in bonding to sulfur, then 58 electrons remain, corresponding to a well-known filled shell. Indeed, a naked cluster in the gas phase containing 58 gold atoms shows exceptional stability [11, 12].
There are a number of connections of the Au102 nanoparticle structure with previous work. First, structures of small gold, silver, and platinum clusters, and of large platinum-palladium clusters, include five-fold symmetry elements and also, in one case, thiols bridging between pairs of gold atoms [13-16]. Second, electron microscopy, X-ray powder diffraction and theoretical studies of large gold clusters have given results consistent with a decahedron [3- 13]. Third, theoretical studies have raised the possibility of distinct gold-sulfur units capping a central gold core [17]. Fourth, the Face centered cubic (fcc) packing in the core, with a gold-gold distance of 2.8-3.1 Å, corresponds with the fcc packing in bulk metallic gold, with a gold-gold distance of 2.9 Å. Fifth, the staple motif, containing alternating gold and sulfur atoms, resembles the gold-thiol polymers believed to represent intermediates in the process of nanoparticle formation [18]. Finally, circular dichroism measurements on gold nanoparticle preparations have shown chiro-optical activity [19].
We have screened fifteen crystals derived from multiple gold nanoparticle preparations and obtained the same gold-102 structure. Other nanoparticle preparations, however, which have also given rise to large single crystals, will doubtless reveal other core structures, from which rules or principles of core assembly may ultimately be derived. It remains to investigate the chemical and physical properties of the Au102 nanoparticle, as well as to explore the theoretical basis of the
- Brust, M. and C.J. Kiely, Monolayer protected clusters of gold and silver. Colloids and Colloid Assemblies, 2004: p. 96-119.
- Daniel, M.-C. and D. Astruc, Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical reviews, 2004. 104(1): p. 293-346.
- Yacaman, M.J., et al., Structure shape and stability of nanometric sized particles. Journal of Vacuum Science & Technology B-an International Journal Devoted to Microelectronics and Nanometer Structures-Processing Measurement and Phenomena, 2001. 19(4): p. 1091-1103.
- Ascencio, J.A., et al., Structure determination of small particles by HREM imaging: theory and experiment. Surface Science, 1998. 396(1-3): p. 349-368.
- Cleveland, C.L., et al., Structural Evolution of Smaller Gold Nanocrystals: The Truncated Decahedral Motif. Physical Review Letters, 1997. 79(10): p. 1873-1876.
- Aiken, J.D. and R.G. Finke, A review of modern transition-metal nanoclusters: their synthesis, characterization, and applications in catalysis. Journal of Molecular Catalysis a-Chemical, 1999. 145(1-2): p. 1-44.
- Martin, T.P., Shells of atoms. Physics Reports-Review Section of Physics Letters, 1996. 273(4): p. 199-241.
- Bau, R., Crystal Structure of the Antiarthritic Drug Gold Thiomalate (Myochrysine): A Double-Helical Geometry in the Solid State. Journal of the American Chemical Society, 1998. 120(36): p. 9380-9381.
- Wan, L.J., et al., Molecular Orientation and Ordered Structure of Benzenethiol Adsorbed on Gold(111). J. Phys. Chem. B, 2000. 104(15): p. 3563-3569.
- Vericat, C., M.E. Vela, and R.C. Salvarezza, Self-assembled monolayers of alkanethiols on Au(111): surface structures, defects and dynamics. Physical Chemistry Chemical Physics, 2005. 7(18): p. 3258-3268.
- de Heer, W.A., The physics of simple metal clusters: experimental aspects and simple models. Rev. Mod. Phys., 1993. 65: p. 611-676.
- Martin, T.P., et al., Shell structure of Clusters. J. Phys. Chem., 1991. 95: p. 6421-6429.
- Mednikov, E.G., M.C. Jewell, and L.F. Dahl, Nanosized (m12-Pt)Pd164-xPtx (CO)72(PPh3)20 (x approximately 7) Containing Pt-Centered Four-Shell 165-Atom Pd-Pt Core with Unprecedented Intershell Bridging Carbonyl Ligands: Comparative Analysis of Icosahedral Shell-Growth Patterns with Geometrically Related Pd145(CO)x(PEt3)30 (x approximately 60) Containing Capped Three-Shell Pd145 Core. J. Am. Chem. Soc., 2007. 129: p. 11619-11630.
- Shichibu, Y., Biicosahedral Gold Clusters [Au25(PPh3)10(SCnH2n+1)5Cl2]2+ (n = 2-18): A Stepping Stone to Cluster-Assembled Materials. Journal of Physical Chemistry C 2007. 111(22): p. 7845-7847.
- Teo, B.K. and H. Zhang, Synthesis and Structure of a Neutral Trimetallic Biicosahedral Cluster, (Ph3P)10Au11Ag12Pt2Cl7. A Comparative Study of Molecular and Crystal Structures of Vertex-Sharing Biicosahedral Mixed-Metal Nanoclusters. Journal of Cluster Science 2001. 12(1): p. 349-383.
- Teo, B.K. and e. al., Rotamerism and roulettamerism of vertex-sharing biicosahedral supraclusters: Synthesis and structure of [(Ph3P)10Au13Ag12Cl8](SbF6). Journal of Cluster Science 1993. 4(4): p. 471-476.
- Hakkinen, H., M. Walter, and H. Gronbeck, Divide and protect: Capping gold nanoclusters with molecular gold-thiolate rings. Journal of Physical Chemistry B, 2006. 110(20): p. 9927-9931.
- Templeton, A.C., W.P. Wuelfing, and R.W. Murray, Monolayer-Protected Cluster Molecules. Accounts of Chemical Research, 2000. 33(1): p. 27-36.
- Schaaff, T.G. and R.L. Whetten, Giant Gold-Glutathione Cluster Compounds: Intense Optical Activity in Metal-Based Transitions. Journal of Physical Chemistry B, 2000. 104(12): p. 2630-2641.
Jadzinsky, P.D., G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution. Science, 2007. 318, p. 430-433.




