The development of bacterial resistance to conventional antibiotics is a major public health concern. For example, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and Staphylococcus aureus (VRSA) have emerged as common nosocomial (hospital-originating) infections. Circumvention of such resistance may be possi ble by emulating host defense antimicrobial peptides (AMP's), which are found in a broad range of species and have broad-spectrum antimicrobial properties. These AMP's have two structural motifs in common: they are cationic and amphipathic. It is thought that electro static interactions facilitate association of the peptide with the anionic bacterial membrane and amphiphilic interactions act to form pores in the bacterial membrane, leading to cell death. Thus, AMP's target generic characteristics common to the membranes of many pathogenic species, and resistance to such natural defences evolves much more slowly than for conventional antibiotics. The exact molecular mechanisms by which membrane pores are formed are still not fully understood, although three major models ('barrel-stave', 'toroidal pore', 'carpet') have been proposed. Moreover, these models do not exhaustively cover all possibilities, as AMP activity is not always correlated with the loss of a permeability barrier. Understanding the structural tendencies generated in antimicrobial-membrane interactions is an essential step to elucidating such molecular mechanisms and therefore to the predictive design of synthetic AMP analogs.
Over the last decade, new peptidic and nonpeptidic antimicrobial oligomers (AMO's) with tunable structural features similar to those of natural, membrane-spanning AMPs have been synthesized and investigated. Like AMP's, these oligomers are also cationic and amphiphilic. However, these synthetic analogs have significantly lower molecular weights, and often do not span the membrane. Synthetic AMO's have the advantage that their structures can be tuned so that the importance of electrostatic and amphiphilic interactions to membrane remodeling and cytotoxicity can be systematically probed.
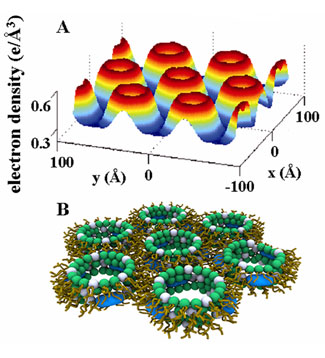
In this work, we use synchrotron small-angle x-ray scattering (SAXS) to examine the structures made by self-assembly of model membrane vesicles and members of a prototypical class of synthetic antimicrobial oligomers (AMO) based on the meta-phenylene ethynylene (mPE) family. These molecules are simple divalent oligomeric amphiphiles. As the hydrophobic volume of these AMO's increases, 3 homologues in this family are inactive (AMO-1), specifically active against bacteria (AMO-2), or non-specifically active against bacteria and eukaryotic cells (AMO-3). We find that the antibacterial activity of these AMOs correlates with their ability to induce an inverted hexagonal (HII) phase when added to concentrated Small Unilamellar Vesicle (SUV) suspensions. This phase consists of columnar arrays of lipid tubes threaded by water channels with diameters of 3.4 nm. When AMO is applied to dilute Giant Unilamellar Vesicle (GUV) suspensions, encapsulated polymers with a radius of gyration less than this diameter leak out whereas larger polymers are retained. These results suggest a common structural tendency in membrane-AMO interactions, which is expressed as an inverted hexagonal phase in bulk membrane systems and as permeated quasi-2D membranes in isolated vesicles. The generation of an HII phase requires negative curvature. This provides a potential mechanism for selective activity: bacterial membranes are rich in PE lipids, which have a negative intrinsic curvature, while eukaryotic cell membranes are rich in PC lipids, which have zero intrinsic curvature. Using concentrated SUV suspensions composed of DOPG:DOPE:DOPC , we find that the inactive AMO-1 cannot generate the inverted hexagonal phase even when DOPE completely replaces DOPC, whereas the specifically active AMO-2 requires a threshold ratio of DOPE:DOPC1 = 4:1. In contrast, the nonspecifically active AMO-3, which lyses eukaryotic cells, requires a drastically lower threshold ratio of DOPE:DOPC = 1.5:1. These trends suggest an explanation for the mechanism of specific activity, since it is well known that the PE content of eukaryotic membranes is significantly lower than that of bacterial membranes.
We use conventional abbreviations for lipids. The first two letters indicate the length of the unsaturated hydrophobic alkyl chain (DO = 18 carbon atoms) and the second two letters indicate the structure of the hydrophilic headgroup: PE = Phosphoethanolamine; PG = Phosphoglycerol; PC = Phosphocholine; PS = Phosphoserine.
L. Yang, V. D. Gordon, A. Mishra, A. Som, K. R. Purdy, M. A. Davis, G. N. Tew and G. C. L. Wong, "Synthetic Antimicrobial Oligomers Induce a Composition-dependent Topological Transition in Membranes", J. Am. Chem. Soc. 129, 12141 (2007)




