 There are over 4,500 known halogenated natural products. The presence of a halogen in the molecular framework tunes a compound's chemical reactivity or biological activity in these natural fungicides and antibiotics. Four classes of enzymes are now known to catalyze halogenation reactions: 1) vanadium haloperoxidases, 2) heme haloperoxidases, 3) flavin-dependent halogenases, and 4) non-heme iron, alpha-ketoglutarate (aKG) dependent halogenases.1,2 Walsh and coworkers first identified the aKG dependent oxygenases in 20053 and noted that they catalyze the insertion of halides into unreactive substrates (for example, the chlorination of a terminal methyl group in barbamide4). The chemical logic of the non-heme iron halogenases follows that of the aKG dependent, non-heme iron dioxygenases such as TauD, the E. coli enzyme catalyzing the hydroxylation of taurine (2-amino-1-ethanesulfonic acid). 5,6 For TauD, a high valent Fe(IV)-oxo species is generated that abstracts a hydrogen atom from the substrate and a subsequent radical rebound results in the hydroxylation of the substrate.7-9 A conserved His-His-Asp/Glu "facial triad" provides the iron ligands.
There are over 4,500 known halogenated natural products. The presence of a halogen in the molecular framework tunes a compound's chemical reactivity or biological activity in these natural fungicides and antibiotics. Four classes of enzymes are now known to catalyze halogenation reactions: 1) vanadium haloperoxidases, 2) heme haloperoxidases, 3) flavin-dependent halogenases, and 4) non-heme iron, alpha-ketoglutarate (aKG) dependent halogenases.1,2 Walsh and coworkers first identified the aKG dependent oxygenases in 20053 and noted that they catalyze the insertion of halides into unreactive substrates (for example, the chlorination of a terminal methyl group in barbamide4). The chemical logic of the non-heme iron halogenases follows that of the aKG dependent, non-heme iron dioxygenases such as TauD, the E. coli enzyme catalyzing the hydroxylation of taurine (2-amino-1-ethanesulfonic acid). 5,6 For TauD, a high valent Fe(IV)-oxo species is generated that abstracts a hydrogen atom from the substrate and a subsequent radical rebound results in the hydroxylation of the substrate.7-9 A conserved His-His-Asp/Glu "facial triad" provides the iron ligands.
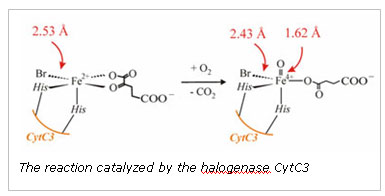
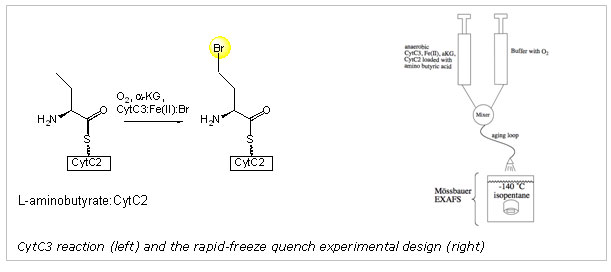
Key insight into the mechanistic differences and similarities of the aKG-dependent halogenases and oxygenases came from the crystal structure of the halogenase SyrB2, responsible for the chlorination reaction in the synthesis of syringomycin, a compound secreted by Pseudomonas syringae.10 The structure indicated that a halogen (Br or Cl) directly binds to the iron in place of the conserved Asp/Glu. Thus, when a ferryl-oxo intermediate is generated upon decarboxylation of the bound aKG, the intermediate abstracts a hydrogen atom from the substrate and a halogen radical is transferred to the substrate radical. Using the halogenase CytC3, an enzyme capable of halogenating L-aminobutyrate, the Bollinger, Krebs, and Walsh groups verified a key component of these proposed mechanistic steps. They demonstrated that a high valent iron intermediate accumulates when the halogenase CytC3 is exposed to oxygen in the presence of Cl-, aKG, and the scaffold enzyme CytC2-substrate complex.11 They furthermore demonstrated that this Fe(IV) species was indeed responsible for hydrogen atom abstraction from the substrate.
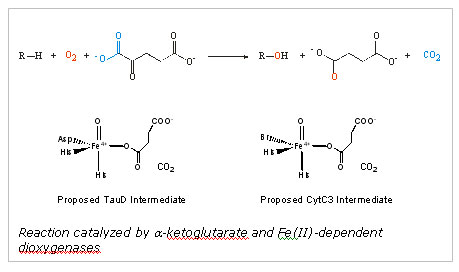
In the present study, X-ray absorption spectroscopy and Mössbauer spectroscopy were used to identify the structural features consistent with the mechanistic model, i.e. the presence of a short Fe(IV)-oxo interaction and a metal bound halide. The Mössbauer spectra collected prior to XAS data collection demonstrated that the quenched samples contained ~80% of the Fe(IV) intermediate. Our XAS spectrum of the intermediate showed a large enhancement in the 1s->3d transition pre-edge intensity relative to the anaerobic, reduced control, which is consistent with the presence of an asymmetrical Fe(IV)= O2- unit. The XANES edge energy is consistent with the 80% Fe(IV): 20% Fe(II) composition determined using Mössbauer spectroscopy. The most compelling evidence for the presence of a formal Fe(IV)=O2- species comes from the fitting analysis of the EXAFS oscillations. Fits to the Fourier-filtered data require a short, 1.62 (± 0.02) Å Fe-O interaction to best model the data. Furthermore, a large scatterer is apparent in the Fourier transformation of the data. This peak can be modeled with an Fe-Br interaction at 2.43 Å. If the coordination number of the short Fe-O interaction and the Fe-Br interactions are systematically varied, the optimal coordination number is 0.7-0.8 for both features, matching the sample composition determined by Mössbauer spectroscopy. In contrast, fits to the reduced control sample were not improved by adding a short Fe-O interaction. The Fe-Br interaction in the control is 2.53 Å, consistent with the distance found in the crystal structure of SyrB2. The structural features we identified using XAS are only consistent with a Br-Fe(IV)=O2- unit and confirms a key component of the proposed mechanism.
- Vaillancourt, F.H., Yeh, E., Vosburg, D., Garneau-Tsodikova, A., and Walsh, C.T. (2006). Chemical Reviews 106, 3364-3378.
- Yarnell, A. (2006). Chemical and Engineering News 84, 12-18.
- Vaillancourt, F.H., Yeh, E., Vosburg, D.A., O'Connor, S.E., and Walsh, C.T. (2005). Nature 436, 1191-1194.
- Chang, Z., Flatt, P., Gerwick, W.H., Nguyen, V.A., Willis, C.L., and Sherman, D.H. (2002). Gene 296, 235.
- Ryle, M.J., and Hausinger, R.P. (2002). Curr. Opin. Chem. Biol. 6, 193-201.
- Krebs, C., Fujimori, D.G., Walsh, C.T., and J. Martin Bollinger, (2007) J. Acc. Chem. Res., 40, 484-492.
- Proshlyakov, D.A.H., T. F.; Monterosso, G. R.; Ryle, M. J.; Hausinger, R. P. (2004). J. Am. Chem. Soc. 126, 1022-1023.
- Price, J.C., Barr, E.W., Glass, T.E., Krebs, C., and Bollinger, J.M., Jr. (2003). J. Am. Chem. Soc. 125, 13008-13009.
- Price, J., Barr, E.W., Tirupati, B., Bollinger, J.M., Jr., and Krebs, C. (2003). Biochemistry 42, 7497-7508.
- Blasiak, L.C., Vaillancourt, F.H., Walsh, C.T., and Drennan, C. (2006). Nature 440, 368-371.
- Galonic, D.P., Barr, E.W., Walsh, C.T., J. Martin Bollinger, J., and Krebs, C. (2007). Nature Chemical Biology 3, 113-116.
Galonic Fujimori, D., Barr, E. W., Matthews, M.L., Koch, G. L., Yonce, J. R.*, Walsh, C. T., Bollinger, J. M., Jr., Krebs, C., Riggs-Gelasco, P. J. "Spectroscopic Evidence for a High-Spin Br-Fe(IV)-Oxo Intermediate in the a-Ketoglutarate-Dependent Halogenase CytC3 from Streptomyces", 2007, J. Am. Chem. Soc., 129, 13408-13409.




