Growth factors are peptides and proteins that function extracellularly to regulate cell growth and differentiation. They play critical roles in vertebrates as they coordinate the actions of cells within the same tissue, or cells in one tissue or organ with those in another. They induce their activities by binding and bringing together cell surface receptors, which typically are comprised of an extracellular domain, a single membrane-spanning domain, and an intracellular domain. The extracellular domains are responsible for recognizing and binding the growth factor, while the intracellular domains, when brought into close spatial proximity, are responsible for activating the downstream machinery that brings about an appropriately tuned cellular response.
Growth factors of the transforming growth factor-beta (TGF-b) superfamily have greatly diversified over the course of evolution, with six such factors in nematodes, nine in fruit flies, and forty-two in humans. They include the ancestral bone morphogenetic proteins (BMPs), which play fundamental roles in embryonic patterning, the closely related growth and differentiation factors (GDFs), which regulate cartilage and skeletal development, the activins, which regulate the release of pituitary hormones, and the evolutionary latecomers, the transforming growth factor-betas (TGF-bs), which regulate cell growth and morphogenesis. BMPs, GDFs, activins, and TGF-bs are homodimers, consisting of two extended monomers held together in most, but not all cases, by an inter-chain disulfide bond.

TGF-bs and related factors induce their response by assembling a heterotetrameric complex comprised of two type I - type II receptor pairs. Type I and type II receptors have the same overall domain structure, including a cysteine rich extracellular domain that adopts a three-finger toxin fold, a single transmembrane helix, and an intracellular serine-threonine kinase domain. The human genome encodes seven type I and five type II receptors. Through cell based crosslinking studies, the BMPs and GDFs have been shown to bind multiple type I and type II receptors in mixed order, while the TGF-bs bind a single type I and a single type II receptor in a pronounced sequential order, first by binding the type II, TbR-II, followed by the type I, TbR-I. These biochemical findings provided the first hint that growth factor receptor complexes of the family might differ structurally.
These differences have been borne out through direct structural analysis of BMP and TGF b:type I receptor:type II receptor ternary complexes. The structure of the BMP ternary complex was first inferred based on independent structures of BMPs bound to BMP type I and type II receptors (Kirsch, et al. and Greenwald, et al., respectively), and was later confirmed by direct analysis of two closely related BMP ternary complexes (Allendorph, et al. and Weber, et al.). The structure of the TGF-b ternary complex, recently reported by Groppe, et al and the subject of this highlight, was determined by direct structural analysis of the TGF b3:TbR I:TbR II ternary complex using a crystal that diffracted to a resolution of 3.0 Å using SSRL beamline 11-1.
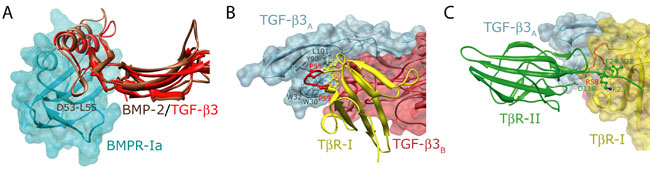
The structures of the ternary complexes reveal that although ligands and receptors of the BMP and TGF-b subfamilies share the same overall fold (Figures 1A and 1B, respectively), they nevertheless bind and assemble their receptors into complexes in ways that are entirely distinct (Figure 1C). The BMP type I and type II receptors bind to the ligand "wrist" and "knuckle" epitopes, respectively, and do not contact one another, while the TGF-b type I and type II receptors bind to the underside of the "fingers" and to the "fingertips", respectively, and have extensive contact. The additional constraints imposed by receptor-receptor contact in the TGF-b, but not the BMP ternary complex, is significant since its accounts for the highly specific and pronounced stepwise manner of receptor binding exhibited by the TGF-bs, but not the BMPs. To demonstrate the functional significance of these, Groppe, et al. substituted a number of critical contact residues in TbR-II, including F24 in the TbR-II N-terminus and D118 near the tip of finger 3, and showed that these impair cooperative assembly in vitro and TGF-b signaling in vivo.
The BMP and TGF-b ternary complexes notably arise from differences in both the manner of type I and type II receptor binding, and hence represent four different modes of binding between molecules that otherwise share the same overall folds (Figure 1A and 1B). This remarkable diversity arises from simple evolutionary modifications of both the ligands and receptors. Thus, as an example, BMPs include a short solvent-exposed helix that binds into a pocket on the BMP type I receptor, BMPR-Ia. This short helical segment in absent in the TGF-bs (Figure 2A), necessitating an alternate manner of type I receptor binding. This is facilitated by a loop, extended in TbRI relative to that in the BMP type I receptors, which binds deeply into a pocket on the underside of the fingers of TGF-b (Figure 2B). This alternate positioning of TbR-I is further supported by an extension of the N-terminal region of TbR-II, which packs against the surface of TbR-I and which fills a hydrophobic pocket on the surface of TbR-I with conserved Phe (F22) and Val (V22) residues (Figure 2C).
In summary, the results recently reported by Groppe, et al. provide a striking example of how simple evolutionary modifications of ligands and receptors of the TGF-b superfamily have enabled alternate modes of receptor complex assembly. It seems likely, based on these observations, that these alternate mechanisms of receptor assembly co-evolved with the TGF-b and BMP specific classes of downstream effectors (Smads) to expand and diversify the role of TGF-b superfamily signaling in vertebrates.
This work was funded by grants from the National Institutes of Health and the Robert A. Welch Foundation (to A. Hinck) and grants from the Canadian Institutes of Health Research and the National Cancer Institute of Canada (to J. Wrana). This study also made use of the Macromolecular Structure Shared Resource of San Antonio Cancer Institute, which is supported by the U. Texas Health Science Center at San Antonio and the National Cancer Institute.
Thomas Kirsch, Walter Sebald, and Matthias K. Dreyer (2000) "Crystal structure of the BMP-2-BRIA ectodomain complex" Nat. Struct. Biol. 7(6):492-496.
Jason Greenwald, Jay Groppe, Peter Gray, Ezra Wiater, Witek Kwiatkowski, Wylie Vale, and Senyon Choe (2003) "The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly" Mol. Cell, 11(3):605-617.
George P. Allendorph, Wylie W. Vale, Senyon Choe (2006). "Structure of the ternary signaling complex of a TGF-beta superfamily member" Proc. Natl. Acad. Sci. U.S.A. 103(20):7643-7648.
Dionys Weber, Alexander Kotzsch, Joachim Nickel, Stefan Harth, Axel Seher, Uwe Mueller, Walter Sebald, and Thomas D. Mueller (2007) "A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor" BMC Struct. Biol. 7:6.
Jay Groppe, Cynthia S. Hinck, Payman Samavarchi-Tehrani, Chloe Zubieta, Jonathan P. Schuermann, Alex B. Taylor, Patricia M. Schwarz, Jeffrey L. Wrana, and Andrew P. Hinck (2008). "Cooperative assembly of TGF-b superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding" Mol. Cell 29(2): 157-168.

