Galactose oxidase is a remarkable metalloenzyme that can selectively oxidize a broad range of primary alcohols to aldehyde without the formation of the thermodynamically more favored carboxylate form. This is done at a Cu containing active site with a cross-linked tyrosine and cysteine ligand, which is formed spontaneously upon binding a Cu(II) ion at the metal site in the presence of dioxygen [1]. The Tyr-Cys crosslink is involved in the catalytic conversion by accommodating one of the electrons from the substrate in addition to the other electron going to the oxidized Cu(II) site. The reduced Cu(I)-O(Tyr-Cys) moiety is then oxidized back to the catalytically active Cu(II)-O(Tyr-Cys) biradical species by dioxygen.
Due to the specificity of the oxidation reaction, the catalytic active site composition and structure have been the focus of numerous biomimetic model studies [2]. It is desirable to synthesize homogeneous catalytic systems that are functional analogues of the GO active site, but neither the Cu(II) site or the separate cross-linked Tyr-Cys radical can achieve the selectivity of GO. The challenge of employing traditional spectroscopic techniques is poised by the diamagnetic electronic structure of the catalytically active form of Cu(II)-O(Tyr-Cys radical), which limits the use of advanced electron paramagnetic resonance techniques to obtain a high resolution, ground state description of the GO active site [3]. Contrary, X-ray absorption spectroscopy can overcome this limitation and provide direct experimental information about the relevant oxidized, semi-reduced, and reduced states of the GO active site.
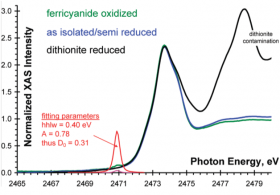
Sulfur K-edge X-ray absorption spectroscopic studies were carried out to directly probe the role of the cross-link in the electronic structure of GO active site. This was possible due to the presence of a thioether bond that is formed in posttranslational modification during the first enzymatic turn-over step. In addition to the low protein concentrations that GO can be purified to without precipitation and the rapid radiation damage during data collection, probing a single sulfur absorber of the thioether bond among 18 other S absorbers from methionine residues and disulfide bridges, with partial Cu(II) loading, and incomplete oxidation state provided an example how far the limits of sulfur XANES can be extended. The intensity of the spectral features assigned to the presence of the thioether bond is small (0.31 eV); however, the spectral feature appeared consistently over repeated data collection.
The S K-edge measurements allowed us to define a significant contribution of the cross-link (24±3%) to the redox active orbital of the Tyr-Cys ligand, which is antiferromagnetically coupled to the electron hole on Cu(II). Follow up density functional theory and high-level correlated ab initio MO calculations allowed the use of this experimental information and evaluate the role of the cross-link on the molecular geometry, reduction potential, optical and magnetic properties. It was concluded that the presence of the cross-link in its immediate protein-environment is critical in fine-tuning the GO active site structure toward the two-electron oxidation of alcohols more efficiently than the Cu(II) carrying out mononuclear radical substitution reaction, which actually is required to form the cross-link during the posttranslational modification.
1. Rogers, M. S.; Baron, A. J.; McPherson, M. J.; Knowles, P. F.; Dooley, D. M. J. Am. Chem. Soc. 2000, 122 (5), 990−991
2. Muller, J.; Weyhermuller, T.; Bill, E.; Hildebrandt, P.; Ould-Moussa, L.; Glaser, T.; Wieghardt, K. Angew. Chem., Int. Ed. 1998, 37(5), 616−619; Pratt, R. C.; Stack, T. D. P. Inorg. Chem. 2005, 44 (7), 2367−2375; Itoh, S.; Taki, M.; Takayama, S.; Nagatomo, S.; Kitagawa, T.; Sakurada, N.; Arakawa, R.; Fukuzumi, S. Angew. Chem., Int. Ed. 1999, 38 (18), 2774−2776
3. Lee, Y. K.; Whittaker, M. M.; Whittaker, J. W. Biochemistry 2008, 47 (25), 6637−6649
Rokhsana D., Howells A.E., Dooley D.M., Szilagyi R.K.: Role of the Tyr-Cys Cross-link to the Active Site Properties of Galactose Oxidase, Inorganic Chemistry, 2012, 51(6), 3513-3524