Almost all organisms require iron as a co-factor in numerous metalloproteins and enzymes. In particular, phytoplankton, which are aquatic, free-drifting, single-celled organisms that can harvest energy from the sun, have an elevated demand for iron due to the large role it plays in their photosynthetic machinery. In 30-40% of the world's oceans iron concentrations are low enough to limit the growth of phytoplankton (Martin and Fitzwater 1988; Moore et al. 2002). New sources of iron to these regions are sporadic and typically include atmospheric dust deposition or weak upwelling of deep waters.
Phytoplankton that persist in these regions must be able to rapidly take up and store iron when it is available and withstand long intervals of limited iron supply. Storing iron in ferritin is one mechanism that enables these organisms to persist through iron droughts. Ferritins are highly specialized iron storage proteins present in many animals, plants and microorganisms. We recently discovered ferritin in a subset of marine diatoms, a highly diverse group of phytoplankton that account for approximately 20% of the total biological uptake of carbon dioxide on the planet (Nelson et al. 1995). The presence of ferritin had not previously been identified within any member of the Stramenopiles, a diverse eukaryotic lineage which includes diatoms and other unicellular algae, macroalgae and plant parasites. Thus far, genes encoding ferritins have only been found in pennate diatoms, a more recent lineage of diatoms that diverged from their centric diatom counterparts about 75 million years ago (Sims et al. 2006).
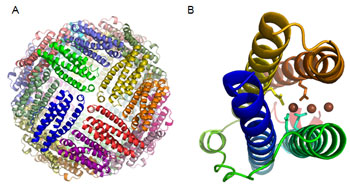
Data collected at SSRL Beam Lines 9.2, 7.1 and at the Canadian Light Source allowed the determination of crystal structures of recombinant ferritin from the marine pennate diatom, Pseudo-nitzschia multiseries (Figure 1). This pennate diatom ferritin forms an assembly of 24 monomers forming a hollow sphere with a diameter of approximately 120 Å, typical of the ferritin family (Figure 2A). In plants and animals, ferritin stores up to 4500 iron atoms as an iron oxide mineral and can release it on cellular demand (Liu and Theil 2005). Cells thereby are protected from potential oxidative damage resulting from the interaction of iron with reactive oxygen. Iron atoms are bound at the ferroxidase centers where ferrous iron is oxidized by oxygen (Figure 2B). The ferroxidase site has unique features suggesting that catalytic activity is tuned for the specific needs of the diatom. Additional iron atoms were found to trail from the ferroxidase center towards the hollow center of the ferritin sphere where mineralization and storage of iron occurs.
The identification of iron as a limiting nutrient to phytoplankton growth has lead to a wave of large-scale iron-enrichment experiments performed in iron-limited regions around the globe. A primary objective of these experiments was to investigate whether more carbon dioxide is removed from the atmosphere when the iron-induced phytoplankton blooms sink to the ocean floor (Boyd et al. 2007). The dominance of ferritin-containing pennate diatoms during most of these experiments is probably not coincidental but rather due in part to high iron storage mediated through ferritin. We found that when placed in an iron-free environment, an oceanic pennate diatom that contains ferritin underwent more cell divisions based on stored iron than an oceanic centric diatom species that putatively does not contain ferritin. The discovery of ferritin in a subset of diatoms helps elucidate how some phytoplankton take advantage of pulsed iron supplies by safely sequestering large amounts of iron that support subsequent growth and divisions well after iron levels return to low, ambient concentrations. This study provides an example of how a single molecule such as ferritin may be influential in defining the success and distributions of ecologically important groups of marine primary producers.
This research was supported by a Gordon and Betty Moore Foundation Marine Microbiology Investigator Award, a National Science Foundation grant, a National Institute of Environmental Health Sciences grant, a National Sciences and Engineering Research Council of Canada grant and a Canadian Institutes of Health Research grant. Portions of this research were carried out at the Canadian Light Source and the SSRL.
Boyd, P. W., T. Jickells, C. S. Law, S. Blain, E. A. Boyle, K. O. Buesseler, K. H. Coale, J. J. Cullen, H. J. W. De Baar, M. Follows, M. Harvey, C. Lancelot, M. Levasseur, N. P. J. Owens, R. Pollard, R. B. Rivkin, J. Sarmiento, V. Schoemann, V. Smetacek, S. Takeda, A. Tsuda, S. Turner, and A. J. Watson. 2007. Mesoscale iron enrichment experiments 1993-2005: Synthesis and future directions. Science 315: 612-617.
Martin, J. H., and S. Fitzwater. 1988. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Nature 331: 341-343.
Moore, J. K., S. C. Doney, D. M. Glover, and I. Y. Fung. 2002. Iron cycling and nutrient-limitation patterns in surface waters of the World Ocean. Deep-Sea Research II 49: 463-507.
Nelson, D. M., P. Treguer, M. A. Brzezinski, A. Leynaert, and B. Queguiner. 1995. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationships to biogenic sedimentation. Global Biogeochemical Cycles 9: 359-372.
Sims, P. A., D. G. Mann, and L. K. Medlin. 2006. Evolution of the diatoms: insights from fossil, biological and molecular data. Phycologia 45: 361-402.
Marchetti A., Parker M.S., Moccia L.P., Lin E.O., Arrieta A.L., Ribalet F., Murphy M.E.P., Maldonado M.T., Armbrust E.V. (2009) Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature, 457, 467-470.




