Funeral practices are accorded high significance in most societies and understanding them hold a key to a much deeper understanding of how those societies functioned. These practices tell us how people from centuries past viewed death and after-life, and therefore give us a glimpse into their existential beliefs. The objects associated in these rituals say something about how they lived and what they valued. Furthermore, because of the importance of these rituals in most societies, the objects involved in them have a much higher chance of survival and are often the only material evidence we have of a culture, a society or a civilization.
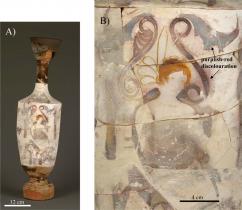
One such object type is the white-ground Lekythos from Attica from the 5th cent BCE. See Fig. 1. The white-ground slips of these oil vessels were painted with depictions of the basic events of the funeralprothesis, procession, inhumation, and subsequent visitation of the grave by the livingas well as the transfigurative journey of the deceased from the world of the living into the afterlife. These painting are often colourful and carefully executed employing a palette of green (malachite, CuCO3and Cu(OH)2), blue (Egyptian blue; mineral cuprorivaite, CaCuSi4O10), red (vermilion, HgS), and orange (iron earths, coloured by Fe2O3) colours. Many of these pigments are fugitive at typical firing temperatures, and thus must have been applied to the vessels after their initial firing. Paintings on Lekythoi provide a primary visual corroboration of some of the descriptions found in ancient Greek mythology and Homeric poems. However, the specific function of these vessels within the funeral ceremony is poorly understood. We present here material evidence that the vessels were ritually burned, together with the body of the deceased, during cremation.
Material evidence for this determination comes from a first systematic chemical and morphological analysis of distinctive purplish-red discolouration that appears as diffuse fields and isolated spatters of colour on the surface of many Attic white-ground lekythoi, and often found adjacent to an area painted by the pigment Egyptian blue. The differential appearance of the discoloration on adjoining sherds across a break on some vessels (see Fig. 1) implies that the vessels were broken prior to the formation of this coloured material. The diffuse and spattered appearance of these purplish-red areas further suggests it was not an intentional element of design.
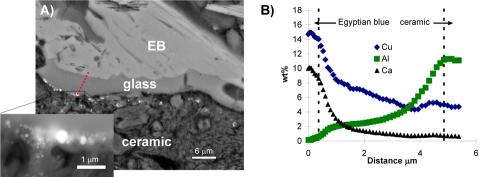
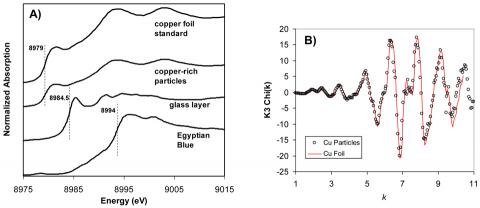
Minute cross-sections were taken from two Lekythoi via a technique perfected for sampling materials from sensitive museum quality objects. The composition and morphology of the purplish-red discolouration was investigated first by electron probe microanalysis (EPMA) and scanning electron microscopy (SEM). Fig 2A, shows a representative cross-section taken from a purplish-red area with overlying Egyptian blue (EB) pigment. There is a thin glass layer sandwiched between the stacked lathes of EB and the ceramic, but significant features in this image are the small, spherical, high Z-contrast nanoparticles lying at and just below the glass/ceramic slip interface, determined by EPMA to be copper-rich. Fig. 2B shows the concentrations of Ca, Al and Cu, the elements that demonstrate the most significant variations, as a function of position. The concentrations of both Cu and Ca are highest in the EB and gradually decrease across the glass layer. The bump in Cu content near the glass-ceramic slip interface corresponds to the location of the Cu-rich particle. By contrast, the Al profile exhibits the opposite trend: the lowest concentration is found in the EB, with a gradual increase across the glass layer, with the highest concentration found in the ceramic slip. These data indicate a bi-directional diffusion: diffusion of Al out of the ceramic layer and counter-diffusion of Ca and Cu from the EB particle into the ceramic matrix underneath. into the glass layer: Al in the ceramic slip has undergone dissolution and diffusion into the glass layer, while Cu and Ca, associated with the EB pigment, have similarly diffused into the glass layer from the pigment layer. These counter diffusion profiles indicate that the Al rich white-ground vessels overpainted with EB and other pigments were subjected to prolonged exposure to high temperature. The Micro x-ray absorption near edge structure (XANES) , shown in Fig 3A, spectroscopy along a similar depth profile indicates that the oxidation state of Cu reduces from +2 to metallic as it diffuse out from EB into Cu rich precipitates just below the surface of the Al rich ceramic. The XANES depth profile, therefore, indicates that not only these vessels were cooked at high temperatures after overpainting, but they were cooked in reducing atmosphere. Extended x-ray absorption fine structure (EXAFS) spectra taken from the Cu-rich nanoparticles, further indicate that these reduced Cu particles are metallic Cu in nature. Light absorption at the surface Plasmon excitation in these metallic Cu nanoparticles gives rise to the purplish discoloration.
The purplish discoloration and uneven coverage over breaks is then a clear signature that these vessels were thrown into a high temperature reducing fire, such as a smoky funeral pyre. And an understanding the origin of the discoloration on these vessels provides the first material evidence that fine ceramicsincluding white-ground lekythoi, typically associated with banquet service, were deliberately broken and then burned as part of a cremation ceremony.
Unfortunately, the majority of lekythoi in museum collections were not archaeologically excavated under controlled conditions, and thus any direct link associating them with a funeral ritual has been lost. However, the evidence presented here provides a new means by which this class of ceramic may be directly linked with the cremation process, thereby enabling archaeologists and art historians to confidently include a much a larger number of these objects in their analysis, which in turn has the potential to dramatically change our understanding of Athenian funerary ritual.
Arletti, R., Dalconi, M.C., Quatieri, S., Triscari, M., Vezzalini, G., 2005. Roman coloured and opaque glass: a chemical and spectroscopic study. Applied Physics A: Materials Science and Processing 83, 239–245.
Gonella, F., Quaranta, A., Padovani, S., Sada, C., D’Acapito, F., Maurizio, C.,Battaglin, G., Cattaruzza, E., 2005. Copper diffusion in ion-exchanged soda-lime glass. Applied Physics A 81, 1065–1071.
Molera, J., Pradell, T., Salvado´ , N., Vendrell-Saz, M., 2001. Interaction between clay bodies and lead glazes. Journal of the American Ceramic Society 84, 1120–1128.
M. S. Walton, M. Svoboda, A. Mehta, S. Webb and K. Trentelman, "Material Evidence for the Use of Attic White-ground Lekythoi Ceramics in Cremation Burials", J. Archaeol. Sci. 37, 936 (2010) doi: 10.1016/j.jas.2009.11.026




