A typical eukaryotic cell contains multiple organelles (nucleus, mitochondria, endoplasmic reticulum, etc.) that have specialized functions. Very often, certain organelles can be found very close to the plasma membrane that surrounds each cell. ‘Junctions’ are parts of the cell where the plasma membrane and the endoplasmic reticulum or the sarcoplasmic reticulum (SR) in muscle cells are in close proximity, about 15 nm apart. This allows for specialized communication between proteins embedded in these different membranes.
Junctophilins (JPHs) are a special class of proteins that directly mediate the formation of junctions in various tissues, including cardiac and skeletal muscle cells, neurons, T-cells, and more1,2. The cardiac isoform is a target for many mutations that cause hypertrophic cardiomyopathy, a condition whereby the heart muscle is abnormally thick, restricting efficient pumping3. Cardiac stress has also been shown to result in proteolytic cleavage of junctophilin, driving the progression of heart failure4-9. Patients with heart failure have reduced exercise tolerance and almost half die of sudden cardiac death, a result of cardiac arrhythmias.
As there were no experimental structures available for JPHs, the Van Petegem group at the University of British Columbia set out to solve crystal structures of the JPH1 and JPH2 isoforms10. Using the macromolecular crystallography beamlines at the SSRL, the group was able to obtain high-resolution diffraction data and to determine experimental phases, which were critical for obtaining the structures.
Fig. 1 shows the peculiar JPH structure for the JPH2 isoform. At its N-terminus, JPH encodes so-called ‘MORN’ repeats (membrane occupation recognition nexus), followed by an α-helical region. The structure resembles a skeleton, whereby the MORN repeats form the ribs, and the long α-helix forms the backbone.
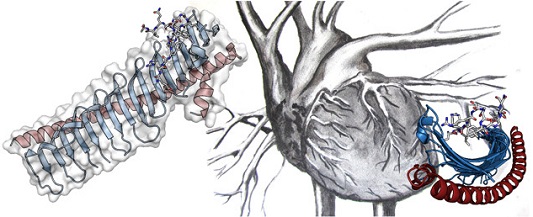
Figure 1: Crystal structure of JPH2, showing the MORN repeats in blue, and the α-helical backbone in red. The sticks represent a portion of the voltage-gated calcium channel (Cav1.1) found in skeletal muscle that was co-crystallized with JPH2. Many different point mutations in JPH2 can cause hypertrophic cardiomyopathy.
In addition to creating junctions, the JPHs have been proposed to bind to various membrane proteins, often modulating their functional behaviour. A prime example of this exists in cardiac and skeletal muscle, where two types of ion channels – voltage-gated calcium channels (CaVs) in the plasma membrane and Ryanodine Receptors (RyRs) in the SR membrane – cooperate for the influx of Ca2+ in the cytosol. In particular, when cardiac or skeletal muscles receive an electrical signal (i.e., a so-called ‘depolarization’ of the plasma membrane), this causes the opening of both CaVs and RyRs, which ultimately results in the release of Ca2+ ions from the SR into the cytosol. These Ca2+ ions then stimulate muscle contraction. The process of converting the electrical signal into a Ca2+ signal is known as ‘excitation-contraction coupling’ and is critically dependent on the proximity between CaVs and RyRs. Thus, JPHs play a key role in the contraction of both cardiac and skeletal muscle.
To better understand their role, the Van Petegem Group investigated how JPHs bind to these channels by solving a structure of a junctophilin in complex with a peptide originating from a muscle ion channel (CaV1.1). The peptide binds to a groove formed by the first three MORN repeats. In collaboration with Wietske Tuinte and Marta Campiglio at the University of Innsbruck, the group probed the importance of the binding interface in muscle cells. They showed that the disruption of the binding resulted in a strong decrease in the muscle excitation-contraction coupling, thus highlighting the role of junctophilins in this process.
In heart muscle, only one JPH isoform is expressed (JPH2). As a result, point mutations in this protein can cause hypertrophic cardiomyopathy. The structures allowed the authors to map more than 80 such mutations on the structure and to analyze their potential impact. They found that many are predicted to stabilize the folding of JPH2. Several of them are located near the interface with the CaV channel and can reduce or even fully disrupt the interaction. These would significantly affect the Ca2+ signaling that’s required for contraction of the heart muscle.
Many questions remain to be answered. Future studies will build on this structural understanding to discover how JPHs interact with other proteins to perform their functions in muscle cells.
- S. E. Lehnart and X. H. T. Wehrens, "The Role of Junctophilin Proteins in Cellular Function", Physiol. Rev. (2022).
- S. Perni, "The Builders of the Junction: Roles of Junctophilin1 and Junctophilin2 in the Assembly of the Sarcoplasmic Reticulum-Plasma Membrane Junctions in Striated Muscle", Biomolecules 12 (2022).
- B. Musumeci et al., "Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge", J. Clin. Med. 10 (2021).
- S. Wang et al., "SERCA2a Ameliorates Cardiomyocyte T-tubule Remodeling via the Calpain/JPH2 Pathway to Improve Cardiac Function in Myocardial Ischemia/Reperfusion Mice", Sci. Rep. 11, 2037 (2021).
- A. Guo et al., "Overexpression of Junctophilin-2 does not Enhance Baseline Function but Attenuates Heart Failure Development after Cardiac Stress", Proc. Natl. Acad. Sci. USA 111, 12240-5 (2014).
- S. K. Lahiri et al., "Nuclear Localization of a Novel Calpain-2 Mediated Junctophilin-2 C-terminal Cleavage Peptide Promotes Cardiomyocyte Remodeling", Basic Res. Cardiol. 115, 49 (2020).
- A. Guo et al., "E-C Coupling Structural Protein Junctophilin-2 Encodes a Stress-adaptive Transcription Regulator", Science 362(2018).
- R. M. Murphy et al., Ca2+-dependent Proteolysis of Junctophilin-1 and Junctophilin-2 in Skeletal and Cardiac Muscle", J. Physiol. 591, 719-29 (2013).
- C. Y. Wu et al., "Calpain-dependent Cleavage of Junctophilin-2 and T-tubule Remodeling in a Mouse Model of Reversible Heart Failure", J. Am. Heart Assoc. 3, e000527 (2014).
- Z. F. Yang et al., Structures of the Junctophilin/Voltage-gated Calcium Channel Interface Reveal Hot Spot for Cardiomyopathy Mutations. Proc. Natl. Acad. Sci. USA 119, e2120416119 (2022).
Z. F. Yang, P. Panwar, C. R. McFarlane, W. E. Tuinte, M. Campiglio and F. Van Petegem, "Structures of the Junctophilin/Voltage-gated Calcium Channel Interface Reveal Hot Spot for Cardiomyopathy Mutations", Proc. Natl. Acad. Sci. USA 119, e2120416119 (2022) doi: 10.1073/pnas.2120416119




