Life is mostly composed of the elements carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorous. Although these six elements make up biomolecules such as nucleic acids, proteins, and lipids, it is theoretically possible that some other elements in the periodic table could serve similar functions. In a paper published in Science, Wolfe-Simon et. al., describe a bacterium of the Halomonadaceae family, strain GFAJ-1 which appears to substitute arsenic for phosphorous to sustain its growth.
Arsenic is a chemical analog of phosphorous, and lies directly below P on the periodic table. Arsenic also possesses a similar atomic radius and has a nearly identical electronegativity as P (1). The most common form of P in biology is phosphate (PO43-) which behaves similarly to the As analog, arsenate (AsO43-) over the range of biologically relevant pH and redox gradients (2). These similarities are thought to contribute to the toxicity of As (3), as As will interfere in the normal metabolic pathways for P. Although As biomolecular analogs of P are believed to be too chemically unstable to be viable (4), given the similarities between As and P, it was hypothesized that some microorganisms may have found a way to cope with and incorporate As into its biomolecules.
To experimentally test this hypothesis, AsO43-, combined with no added PO43-, was utilized to apply selective pressure to isolate such a microbe. Sediments from Mono Lake, located in eastern California, were used as inocula into an aerobic media at pH 9.8, containing glucose, vitamins, and trace metals. Neither PO43- nor any complex organic supplements (e.g. yeast extract, peptone) were added. Gradually, AsO43- concentrations were increased, from 100µm to 5mM through successive dilution transfers. After several dilutions and isolation on agar media, the isolate, GFAJ-1, was identified as by 16S rRNA sequence phylogeny as a member of the Halomonadaceae family of Gammaproteobacteria and was maintained aerobically on a media of glucose and 40 mM AsO43-. While GFAJ-1 exhibits a growth rate of 0.53 day-1 under +As/-P conditions, it does show more favorable growth when PO43- is added (0.86 day-1). However, no growth was observed if neither AsO43-nor PO43- were added.
Arsenic content by ICP-MS showed that the intracellular As content of +As/-P cells was ~0.2% by dry weight, with only 0.02% P. This P content is more than 30 times less when grown in the -As/+P condition. Radiolabelled 73AsO43- was used to examine where arsenate was incorporated inside the microbe. Intracellular As was observed in protein, metabolite, lipid, and nucleic acid cellular fractions. Stationary phase cells incorporated approximately 10% of the 73AsO43- label into nucleic acids, and more than 75% of the label into the phenol extracted protein fraction, with a small fraction going into lipids. The protein fraction is likely to include small, non-proteinaceous metabolites as well. High resolution secondary ion mass spectrometry (NanoSIMS) at LLNL positively identified As in extracted, gel purified bands of genomic DNA. These results showed that DNA from +As/-P cells has elevated As and low P relative to DNA from -As/+P cells.
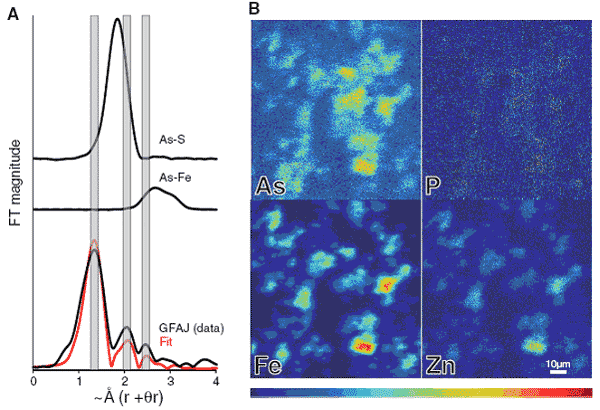
At SSRL, synchrotron x-ray studies were used at BL2-3 to determine the speciation and chemical environment of intracellular As. Micro x-ray fluorescence confirmed ICS-MS results, showing low background P concentrations, and location of relatively high As that spatially correlated with other trace metal elements in cellular material, such as Fe and Zn. Micro x-ray absorption spectroscopy (µXAS) of whole cells from the +As/-P growth condition were examined and exhibited x-ray absorption edge characteristics of As(V). µEXAFS of the cells show As coordinated by 4 oxygen atoms at 1.73 Å, consistent with As(V). Further observed coordination shells were fit with As-C (2.5 at 2.35 Å and 2.2 at 2.92 Å), and were inconsistent with As-Fe and As-S models, free arsenate ions, or published spectra for organo-arsenicals. While other compounds, such as dimethylarsinate (DMA) have As-O and As-C bonds, they have distinctly different As edge positions as well as shorter observed As-O and As-C bond distances. The µEXAFS results represent an average coordination of the As in the whole cells, and are likely to consist of a combination of many As compounds. However, the bond distances observed in the As-C GFAJ-1 cells are consistent with an interpretation of As substituted for P in reported structures for DNA as well as other biomolecules.
These combined results show As-dependent growth by GFAJ-1 in the absence of P. Growth was furthermore accompanied by As uptake and assimilation into cells and biomolecules. These results are different from other organisms which use dissimilatory As uptake to use AsO43- as a terminal electron acceptor for energy production. The discovery of this unusual microbe is exceptional in its ability to substitute As for P in its growth. Future work is in progress to further characterize the stability and ultimate location of As in the biomolecules.
(1) D. Lide, Ed., CRC Handbook of Chemistry and Physics, 90th Edition (Internet Version 2010), CRC Press/Taylor and Francis, Boca Raton, 2010.
(2) F. Wolfe Simon, P.C.W. Davies, A.D. Anbar, Int. J. Astrobiol., 8, 69 (2009).
(3) B. Rosen, FEBS Lett, 529, 86 (2002).
(4) C.D. Baer, J.O. Edwards, P.H. Rieger, Inorg. Chem., 20, 905 (1981).
F. Wolfe-Simon, J. S. Blum, T. R. Kulp, G. W. Gordon, S. E. Hoeft, J. Pett-Ridge, J. F. Stolz, S. M. Webb, P. K. Weber, P. C. W. Davies, A. D. Anbar and R. S. Oremland, "A Bacterium that can Grow by Using Arsenic Instead of Phosphorus", Science(2010) doi: 10.1126/science.1197258 [PMID: 21127214]




