Human and other animals rely on the senses of touch and hearing to perceive mechanical stimuli, a process known as mechanosensation. Bacteria also have the ability to sense mechanical forces through mechanosensitive channels located in their plasma membrane. These channels open and close in response to membrane tension and serve as "pressure relief valves" that protect bacteria from bursting due to the influx of water during osmotic down-shock conditions, as when a bacterium suddenly finds itself surrounded by freshwater. Different types of mechanosensitive channels are present that gate at different pressure thresholds, including the mechanosensitive channel of large conductance (MscL) that opens, or gates, at tensions close to the lytic limit of bacterial cells1. Using data collected at SSRL, the original crystal structure of a MscL homolog from Mycobacterium tuberculosis (TbMscL) was determined in 1998 at 3.5 Å resolution, representing a closed state conformation of MscL2. During the transition from closed to open states, MscL goes through several intermediate states, including one putative expanded non-conductive intermediate and at least three sub-conducting states3.
After 11 years of pursuing MscL structures in different conformational states, Liu et al. have recently determined the structure of a C-terminal truncation mutant of Staphylococcus aureus MscL (SaMscL-Cdelta26) at 3.8 Å resolution, by the method of multiple isomorphous replacement with anomalous scattering, using diffraction data collected at SSRL4. The SaMscL-Cdelta26 crystals have high solvent content (~70%), exhibited high mosaicity and diffracted X-ray weakly beyond 5 Å resolution. The automatic crystal-mounting and data-collecting systems at SSRL enabled the efficient screening of crystals to identify the best diffracting ones. Furthermore, the small beam size on BL12-2 allowed testing different spots of each individual crystal for the collection of one best dataset with high signal-to-noise ratio and low error.
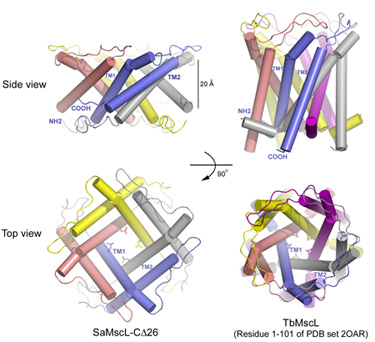
The SaMscL-Cdelta26 structure has several distinct features as compared to the previous TbMscL structure. Firstly, it forms a tetrameric channel instead of a pentameric one as observed for TbMscL previously (Fig. 1). Nevertheless, the general architectures of both channels are similar. Each subunit contains two long transmembrane helices (TM1 and TM2) with TM1 lining the inner surface of the channel lumen and TM2 flanking at the periphery. Both channels have conserved hydrophobic constriction at Val 21 and Leu 17. Secondly and more interestingly, SaMscL-Cdelta26 tetramer is ~13 Å thinner along the membrane normal, but up to 17 Å wider on the periplasmic surface as compared to TbMscL pentamer. Although the constriction at Val 21 is widened to ~6 Å across, about 3 Å larger than the same site in TbMscL, theoretical studies5 suggest that the new structure is still likely to be in a non-conductive state. Meanwhile in SaMscL-Cdelta26, the tilt angles of TM1 and TM2 from the pore axis were dramatically increased to a degree close to the angles described in the open-state models of E. coli MscL6, 7. Consequently, the SaMscL-Cdelta26 structure with pre-expanded conformation is presumably an intermediate state between the closed and open states. Based on a comparison of the MscL structures, a two-step helix pivoting model of the gating mechanism was proposed to account for this intermediate state as a turning point during the gating transition.
This work was supported in part by grants from the Howard Hughes Medical Institute and the National Institutes of Health (GM084211). C.S.G. was supported in part by postdoctoral fellowships from the National Institutes of Health and the Beckman Foundation. Construction of BL12-2 at SSRL was supported by a generous gift from the Gordon and Betty Moore Foundation to the California Institute of Technology.
- Sukharev, S.I., Blount, P., Martinac, B., Blattner, F.R. & Kung, C. Nature 368, 265-268 (1994).
- Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. Science 282, 2220-2226 (1998).
- Sukharev, S. I., Sigurdson, W. J., Kung, C. & Sachs, F. J. Gen. Physiol. 113, 525-540 (1999).
- Liu, Z., Gandhi, C.S. & Rees, D.C. Nature 461, 120-124 (2009).
- Beckstein, O. & Sansom, M. S. P. Phys. Biol. 1, 42-52 (2004).
- Sukharev, S., Betanzos, M., Chiang, C.-S. & Guy, H. R. Nature 409, 720-724 (2001).
- Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A. & Martinac, B. Nature 418, 942-948 (2002).
Zhenfeng Liu, Chris S. Gandhi and Douglas C. Rees "Structure of a tetrameric MscL in an expanded intermediate state". Nature, 461, 120-124 (2009).




