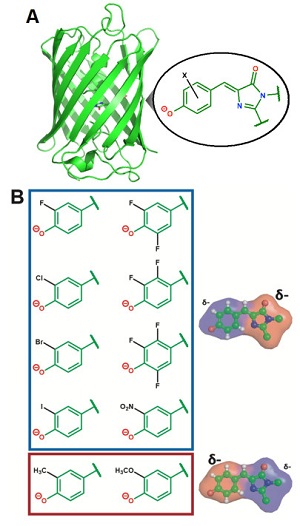
Figure 1. A) Structure of a green fluorescent protein highlighting the chromophore. B) Chemical substituent series designed to systematically perturb the electron distribution within the chromophore.
Light is the most abundant natural resource. Organisms have evolved the ability to take advantage of light as an environmental cue, such as vision in the eyes of animals, phototaxis in both prokaryotes and eukaryotes, and light-dependent signaling pathways to control various processes such as plant germination and growth. Inspired by these processes, scientists have developed light-sensitive molecular tools over the past decade that can control neuronal activity and other cellular signaling pathways of interest within organisms in a light-dependent manner.1 Materials have been engineered to change properties and even store information in response to light using similar principles. At the heart of all these biological and non-biological applications is a molecular process that converts light energy into motion: photoisomerization, or rotation about a double bond upon exposure to light.2
Despite the ubiquity and general utility of photoisomerization, mysteries still remain, especially when considering how to rationally design light-sensitive tools catered towards specific applications using simple guidelines. For example, the key light-responsive molecule, called a chromophore (derived from Greek meaning color-bearing), can undergo a variety of different transformations, including photoisomerization, upon light irradiation.3 The probability of each outcome heavily depends on the chromophore’s environment, and the precise role of the environment in governing these outcomes is not well understood. A research team at Stanford composed of Chemistry graduate students Matthew Romei and Chi-Yun Lin, Professor Steven Boxer, and SSRL Staff Scientist Irimpan Mathews sought to identify the dominant factors that control photoisomerization.
To obtain the underlying physical principles, the researchers focused on a green fluorescent protein that contains a photoisomerizable chromophore (Figure 1A). This protein is an ideal model system since its chromophore is embedded in a well-defined protein environment, and any modifications to the system can be precisely characterized through structural methods. Romei et al. made a series of protein variants by introducing chemical substituents with unnatural amino acids through a technique called amber suppression4 (Figure 1B). This series was designed to subtly yet systematically perturb the electron distribution within the chromophore while keeping all other variables constant (e.g. atomic coordinates), which was confirmed from x-ray crystal structures obtained at SSRL showing little to no structural changes across the variants, even in the immediate environment of the chromophore. Spectroscopic studies on the series of variants revealed a fascinating conclusion: subtle changes in the electron distribution of the chromophore can tune the photoisomerization mechanism by either raising or lowering the energy barrier heights of different bond rotation pathways (Figure 2).
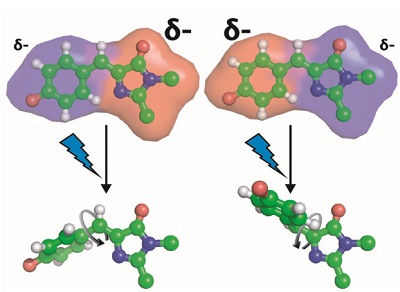
Figure 2. Modification of the chromophore’s electron density alters the photoisomerization pathway, specifically which bond rotates upon light exposure.
These findings suggest that the electrostatics of a chromophore’s environment play an important role in photoisomerization. Since the rotation of different bonds leads to different charge redistribution within the chromophore, tuning the electrostatic environment around the chromophore can bias one photoisomerization pathway over another. Insights gained from these results can aid in the experimental and computational design and engineering of new and exciting light-sensitive tools.
- C. P. O’Banion, D. S. Lawrence, “Optogenetics: A Primer for Chemists”, ChemBioChem 19, 1201 (2018).
- S. Gozem, H. L. Luk, I. Shapiro, M. Olivucci, “Theory and Simulation of the Ultrafast Double-bond Isomerization of Biological Chromophores”, Chem. Rev. 117, 13502 (2018).
- A. Acharya, A. M. Bogdanov, B. L. Grigorenko, K. B. Bravaya, A. V. Nemukhin, K. A. Lukyanov, A. I. Krylov, “Photoinduced Chemistry in fluorescent Proteins: Curse or Blessing?”, Chem. Rev. 117, 758 (2017).
- D. D. Young, P. G. Schultz, “Playing with the Molecules of Life”, ACS Chem. Biol. 13, 854 (2018).
M. G. Romei, C.-Y. Lin, I. I. Mathews and S. G. Boxer, "Electrostatic Control of Photoisomerization Pathways in Protein", Science 367, 76 (2020) doi: 10.1126/science.aax1898




