Controlled and stable electrical doping of organic semiconductors is desirable for the realization of efficient organic photovoltaic (OPV) devices. Thus, progress has been made to understand the fundamental doping mechanisms.1-3 In 2016, Aizawa et al. reported the use of 12-molybdophosphoric acid hydrate (PMA) to induce p-type doping and crosslinking of neat films of poly[N-9’-heptadecanyl-2,7-carbazole-alt-5,5-(4’,7’-di-2-thienyl-2’,1’,3’-benzothiadiazole)](PCDTBT).4 Later on, a more general approach of sequential solution-based doping was presented, by post-process immersion of donor-like polymer films in PMA-nitromethane solutions.5 However, critical to the method is the use of nitromethane, a highly unstable solvent, to dissolve PMA and thus limited the applicability to large-scale fabrication of organic solar cells.
A collaboration between a team of researchers from the Kippelen Research Group at Georgia Tech and the Toney Research Group at SSRL developed a solution-based doping method using the highly stable solvent, acetonitrile. Figure 1a displays the chemicals used in this work. In Figure 1b, the optical properties of poly(3-hexylthiophene-2,5-diyl)(P3HT) films immersed for 30 min in a 0.5 M solution of PMA in acetonitrile (PMA-im-P3HT) were studied by comparing their transmittance spectra against pristine P3HT and P3HT immersed similarly in a 0.5 M solution of PMA in nitromethane. The normalized change of transmittance ΔT T-1 as a function of wavelength (inset of Fig.1b) reveals the same spectral signatures reported for PMA-im-P3HT films when PMA was dissolved in nitromethane. That is, changes in the region where ΔT T-1 < 0 correlate with the P3HT polaron bands, and deviations in the region where ΔT T-1 > 0 correlate to the bleaching of the main π-π* absorption bands.6 The data suggests electrical p-doping into the depth of the organic film. Figure 1c shows that the performance of PMA-doped OPV devices using PMA in acetonitrile is comparable to that of OPVs made using PMA in nitromethane or MoO3, under simulated AM 1.5G solar illumination. Furthermore, if the light soaking mechanism is used before each measurement, OPVs made using PMA in nitromethane or acetonitrile remain stable for up to 524 h in the air, retaining 80% of their initial power conversion efficiency (PCE).
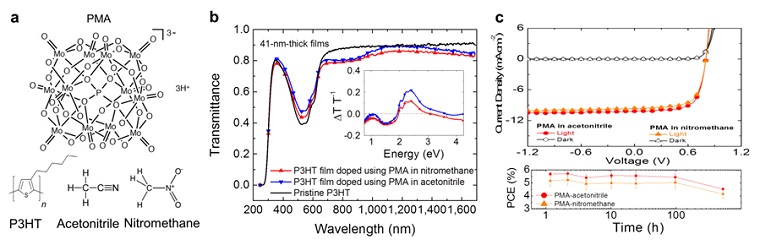
Figure 1. Optical and electrical properties of P3HT films immersed in a PMA solution in acetonitrile or nitromethane. a, Chemical structures of PMA, nitromethane, acetonitrile and P3HT. b, The transmittance of pristine P3HT and PMA-im-P3HT (after post-process immersion in nitromethane or acetonitrile) films, with the normalized change in the transmittance data in the inset. c, Direct comparison of J-V characteristics measured in the dark and under 1-sun illumination and stability test for PMA-im-P3HT:ICBA OPVs exposed to air at room temperature. All measurements were conducted after 10 min soaking under 1-sun illumination in a N2-filled glovebox.
Acetonitrile, nitromethane, and ethanol were used to dissolve the polyoxometalate and the morphology of PMA-im-P3HT films fabricated on Si substrates was analyzed using grazing-incidence wide-angle x-ray scattering (GIWAXS). As expected, the images show that the preferred orientation of P3HT on the SiO2 surface is edge-on, with distinctive (100), (200) and (300) lamellar peaks observed in the out of plane direction with q values of 0.37 Å-1, 0.77 Å-1 and 1.14 Å-1 (Fig. 2b). Also as expected, there is a clear peak on the (010) direction with a q value of 1.68 Å-1 in the in-plane line profile (d-spacing ca. 0.37 nm), which is due to the π-π stacking of P3HT.

Figure 2. GIWAXS data as measured on pristine and PMA doped P3HT, when using various solvents to dissolve the PMA. a, Two-dimensional GIWAXS data converted to q-space for pristine P3HT and P3HT immersed in PMA solutions in nitromethane, acetonitrile or ethanol for 60 seconds. b, One-dimensional scattering profiles (out-of-plane and in-plane profiles), obtained from the two-dimensional GIWAXS data.
Interestingly, the PMA doping does not appear to alter the location of the peaks in the in-plane line profile, which implies no change of the π-π stacking distance of P3HT. Instead, additional peaks appear in the out-of-plane line profile of P3HT samples doped with PMA, when the polyoxometalate is dissolved in either nitromethane or acetonitrile, pointing out to the intercalation of doping molecules between P3HT lamella. Specifically in doped samples using PMA in nitromethane or acetonitrile, near the (200) diffraction, the original neat P3HT peak at 0.77 Å-1 of the out-of-plane line profile appears next to a new peak at 0.69 Å-1. Moreover, near the (300) diffraction, there is a new peak at 1.03 Å-1, in addition to the original peak at 1.14 Å-1. Thus, the GIWAXS data shows that the dopant molecules, when using PMA dissolved in nitromethane or acetonitrile, become intercalated between the lamellar while producing no distortion in the π-π stacking of P3HT. However, such effect is not present when immersing P3HT films into PMA in ethanol. This is consistent with the less effective doping of the PMA-ethanol solution as observed in the past.
In conclusion, the research team reports on the use of acetonitrile, a solvent that is stable in air, as an alternative to nitromethane to enable PMA-based electrical doping of organic semiconductors within a limited depth from the surface. The morphology of doped organic films (using PMA dissolved in nitromethane or acetonitrile) was studied using GIWAXS and the data revealed that the dopant molecules intercalate between the P3HT lamella but cause no change in the π-π stacking. Based on these findings, they propose an explanation to the observed solvent-selectivity of the PMA doping method. Finally, they validated the use of acetonitrile to fabricate solar cells and discovered that these devices showed increased stability when exposed to atmosphere conditions, compared to reference devices fabricated using nitromethane.
F. A. Larrain Benavides, C. Hernandez, W.-F. Chou, V. Rodriguez-Toro, T.-Y. Huang, M. F. Toney and B. Kippelen, "Stable Solvent for Solution-based Electrical Doping of Semiconducting Polymer Films and Its Application to Organic Solar Cells", Energy Environ. Sci. (2018) in press, doi: 10.1039/C8EE00811F




