Imagine being born with severe muscle weakness. Several of your joints are contracted, your spine is abnormally curved, and you have an opening in the roof of your mouth, affecting your hearing, breathing and speech. On top of that, if you undergo surgery, you may die from a serious reaction to the anesthetics that causes your body temperature to rise to lethal levels. This happens to people that have Native American Myopathy (NAM), a disorder first described for the Lumbee Native Americans in North Carolina, where about 1 in 5000 individuals is affected. The cause for the disorder is genetic: a mutation in a gene known as stac3.
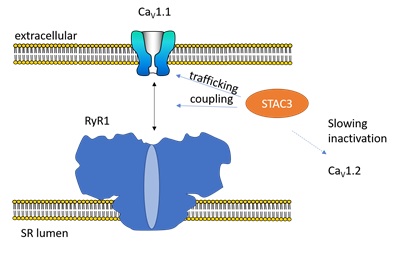
To understand NAM, one needs to look at one of the basic components in skeletal muscle. Just prior to the muscle contraction, an electrical signal is generated in the plasma membrane, causing it to depolarize. Immediately after, Ca2+ ions are being released from the Sarcoplasmic Reticulum (SR), a specialized compartment present in muscle cells. These Ca2+ ions then bind to muscle-specific proteins, ultimately resulting in contraction. Key in this process is the ability of the plasma membrane depolarization to be coupled to Ca2+ release. Also known as ‘excitation-contraction (EC)-coupling’, this process involves multiple components (Figure 1). Voltage-gated calcium channels (CaV1.1), present in the plasma membrane, sense the electrical field and undergo a conformational change. This is then transmitted mechanically to the Ryanodine Receptor (RyR1), another Ca2+ channel that is located in the SR. RyR1 opens and allows Ca2+ to escape into the cytosol of the cell.
How exactly CaV1.1 and RyR1 (the skeletal muscle isoforms) couple has remained a mystery for several decades. Do they form direct interactions, or do they make use of intermediary proteins? Recent reports have shed some much-needed light on this process. The protein STAC3 was found to play three important roles: 1) it is essential for the mechanical coupling of CaV1.1 and RyR1, 2) it helps in bringing the CaV1.1 channels to their final site in the muscle cells, and 3) it slows down inactivation of different CaV isoform, thus making their Ca2+ currents last longer 1-7. Importantly, when this protein is targeted by a mutation (W284S) it causes NAM, further highlighting that it is a crucial component.

Figure 2. a. Schematic outline of the STAC domains, and our crystal structure of the tandem SH3 domains of STAC2. The sticks represent a peptide from the cytosolic II-III loop of CaV1.1. The position of the NAM disease mutation in STAC3 is indicated in black (W284S) b. ITC experiment showing binding of STAC3 to the core II-III loop with a Kd~0.8 μM.
Researchers at the Life Sciences Centre, University of British Columbia and the Medical University of Innsbruck, Austria investigated the 3D structure of STAC3, revealing how it interacts with CaV1.1. In order to facilitate this, the team considered multiple STAC isoforms (STAC1, STAC2, STAC3), since these are all highly similar and can all interact with CaV1.1. The STAC proteins all contain an N-terminal C1 domain, and two SH3 domains at the C-terminus. They found that the SH3 domains form a binding site for a cytosolic loop of CaV1.1, with a Kd ~1 μM. Using macromolecular crystallography beam lines at SSRL, experiments were carried out on crystals prepared with and without the cytosolic loop8. Figure 2 shows the structure, with sticks indicating the cytosolic CaV1.1 loop. The first SH3 domain (SH3-1) forms the bulk of the interactions. Crucial here is a Trp residue, which is the target for the NAM mutation in STAC3 (W284S); the cytosolic loop wraps around this residue, making both hydrophobic and ionic interactions with the SH3 domain. The interaction is lost when the mutation is introduced.
In order to verify the importance of these findings, the team set out to do functional experiments and showed that disrupting the interaction through these mutations in the CaV1.1 loop, the EC-coupling was abolished.
Although this structure now provides a snapshot into the molecular interactions that underlie EC-coupling, the story is far from complete. Does STAC3 also bind to RyR1, thus forming a ‘glue’ that holds CaV1.1 and RyR1 together? Are there any other binding sites in CaV1.1? The latter is almost certain to be true, because the same mutations that knock out EC-coupling do not affect the ability of STAC proteins to slow down inactivation of other CaV isoforms. Indeed, recent reports have suggested that the C1 domain may be involved in this process, forming an interaction with an as-of-yet unidentified site in the CaV channel. More structural studies will therefore be required.
- A. Polster, S. Perni, H. Bichraoui and K. G. Beam, "STAC Adaptor Proteins Regulate Trafficking and Function of Muscle and Neuronal L-type Ca2+ Channels", Proc. Natl. Acad. Sci. USA 112, 602 (2015).
- A. Polster, B. R. Nelson, E. N. Olson and K. G. Beam, "STAC3 has a Direct Role in Skeletal Muscle-type Excitation-contraction Coupling that is Disrupted by a Myopathy-causing Mutation. Proc. Natl. Acad. Sci. USA 113, 10986 (2016).
- B. R. Nelson et al., "Skeletal Muscle-specific T-tubule Protein STAC3 Mediates Voltage-induced Ca2+ Release and Contractility", Proc. Natl. Acad. Sci. USA 110, 11881 (2013).
- E. J. Horstick et al., "Stac3 is a Component of the Excitation-contraction Coupling Machinery and Mutated in Native American Myopathy", Nat. Commun. 4, 1952 (2013).
- M. Campiglio and B. E. Flucher, "STAC3 Stably Interacts through Its C1 Domain with CaV1.1 in Skeletal Muscle Triads", Sci. Rep. 7, 41003 (2017).
- S. Perni, M. Lavorato and K. G. Beam, "De novo Reconstitution Reveals the Proteins Required for Skeletal Muscle Voltage-induced Ca2+ Release", Proc. Natl. Acad. Sci. USA 114, 13822 (2017).
- M. Campiglio et al., "STAC Proteins Associate to the IQ Domain of CaV1.2 and Inhibit Calcium-dependent Inactivation", Proc. Natl. Acad. Sci. USA (2018).
S. M. W. K. Yuen, M. Campiglio, C.-C. Tung, B. E. Flucher and F. Van Petegem, "Structural Insights into Binding of STAC Proteins to Voltage-gated Calcium Channels", Proc. Natl. Acad. Sci. USA 114, E9520 (2017) doi: 10.1073/pnas.1708852114




