Influenza also called “Flu” is a disease of the human respiratory tract caused by influenza virus. Each year, seasonal influenza can cause severe and widespread disease in the human population and cost billions of dollars to the world economy. Such a problem occurred this year with the influenza A H3N2 virus. Currently available remedies to tackle influenza are the seasonal trivalent or tetravalent vaccines or FDA-approved antiviral drugs, such as Tamiflu and Relenza. Since influenza viruses are constantly mutating and circulating as new strains in the human population, influenza vaccines need to be updated each year. Furthermore, the efficacy of these currently available front-line drugs are declining due to the relentless evolution in the influenza virus strains (1-3). Therefore, there is an urgent need for universal influenza therapeutics with novel mechanisms of action and novel targets to tackle the threats posed by influenza viruses.
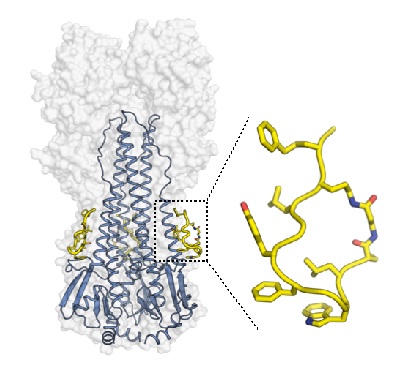
A collaboration between a team of researchers from Janssen Research & Development (Janssen, Netherlands) led by Dr. Maria van Dongen and The Scripps Research Institute led by Prof. Ian A. Wilson developed potent peptidic molecules based on broadly neutralizing antibodies (bnAbs) CR9114 and FI6v3 that target the highly conserved stem epitope on influenza virus hemagglutinin (HA). The designed peptides show high affinity and neutralization against a broad set of group 1 influenza A viruses (H1, H2, H5 and H6 subtypes), including the 2009 H1N1 pandemic and an avian H5N1 flu strain. The peptides are non-toxic, show stability to proteolysis, and have drug-like properties when evaluated in mice.
To investigate their mechanism of action, Dr. Rameshwar Kadam in the Wilson group determined high resolution crystal structures of the peptides in complex with hemagglutinin from A/Puerto Rico/8/1934 (H1N1) (H1/PR8). The x-ray data were collected at SSRL BL12-2. The peptides target a highly conserved pocket in the HA stem region (Figure 1) and mimic the binding mode of bnAbs CR9114 and FI6v3 on HA. The peptides stabilize the HA trimer and prevent the low pH-induced conformational changes in the endosome that are required for fusion of viral and host cell membranes to initiate influenza infection. This structural information on the peptides-HA interaction provides valuable insights for development of the next generation of peptide and small molecule-based therapeutics targeting influenza virus hemagglutinin.
1. R. A. Bright, D. K. Shay, B. Shu, N. J. Cox, A. I. Klimov, "Adamantane Resistance among Influenza A Viruses Isolated Early during the 2005-2006 Influenza Season in the United States", JAMA 295, 891 (2006).
2. A. Moscona, "Global Transmission of Oseltamivir-resistant Influenza", N. Engl. J. Med. 360, 953 (2009).
3. T. G. Sheu et al., "Dual Resistance to Adamantanes and Oseltamivir among Seasonal Influenza A(H1N1) Viruses: 2008-2010", J. Infect. Dis. 203, 13 (2011).
R. U. Kadam, J. Juraszek, B. Brandenburg, C. Buyck, W. B. G. Schepens, B. Kesteleyn, B. Stoops, R. Vreeken, J. Vermond, W. Goutier, C. Tang, R. Vogels, R. H. E. Friesen, J. Goudsmit, M. J. P. van Dongen and I. A. Wilson, "Potent Peptidic Fusion Inhibitors of Influenza Virus", Science 358, 496 (2017) doi: 10.1126/science.aan0516




