The strong electron correlations in transition metal oxides give rise to such phenomena as high-temperature superconductivity in layered cuprates and to stripe-like order in layered cuprates and nickelates. In the case of the manganites, an additional strong electron-lattice interaction leads to a very rich phase diagram in which structural, magnetic, and transport properties are intimately related. Colossal magnetoresistance (CMR) has been observed in the perovskite and double-layer manganites, but not in the single-layer system La1-xSr1+xMnO4 (Mn214). Nevertheless, there are signs that the physics of Mn214 is similar to that of the perovskites. Information about the low-temperature structural phases of Mn214 can be expected to provide valuable insight into the role of dimensionality on the properties of the manganites, and also to contribute to a deeper understanding of single-layer transition metal oxides in general.
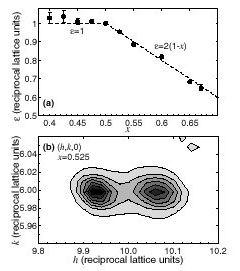
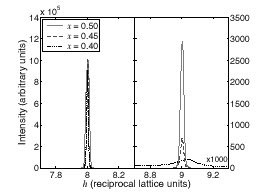
Simon Larochelle and co-workers have grown single crystals at Stanford's new Laboratory for Advanced Materials and carried out x-ray scattering studies at SSRL Beamline 7-2 to establish the low-temperature structural phase diagram of Mn214 [1]. For x = 1/2, this study provides a more complete picture than previous neutron [2] and x-ray [3] scattering experiments. An investigation of the effects of varying the eg electron concentration (ne= 1 - x) in the MnO2 layers revealed three distinct regions: disordered (x < 0.4), mixed-phase (0.4 < x < 0.5), and charge-ordered (x >0.5). Above x = 0.5, the ordering of eg electrons is found to result in a structural distortion whose modulation period only depends on ne. Even though Mn214 does not exhibit CMR, this trend resembles findings for La1-xCaxMnO3, which is a CMR material. This behavior furthermore is reminiscent of the charge- and spin-density wave order tendencies in the hole-doped layered cuprates and nickelates. The results of this study thus provide valuable quantitative information for tests of theories for CMR materials and layered transition metal oxides.
- S. Larochelle et al., Phys. Rev. Lett. 87, 095502 (2001).
- B. J. Sternlieb et al., Phys. Rev. Lett. 76, 2169 (1996).
- Y. Murakami et al., Phys. Rev. Lett. 80, 1932 (1998).




