
The chemical speciation of potentially hazardous subsurface contaminants is a crucial parameter in environmental risk assessment and remediation because it determines their transport, toxicology, and ultimate disposition. This tenet especially applies to uranium and plutonium contamination that arguably constitute the most intractable environmental restoration problems at legacy sites from nuclear weapons production and testing, as well as weapons and reactor accidents. These hazards are further amplified by the public perception of radioactive elements in general, and Pu in particular. The chemical transformations of U after its release is, however, an exceedingly complicated problem, both thermodynamically and kinetically, with less being known about Pu because there are no natural systems to study.
Computer models to describe kinetic and thermodynamic chemical transformations are still constrained to describe these species in the context of known, crystalline compounds. After examining U and Pu in soil samples from six different locations in the U.S. and Russia, some in bulk but most as single particles, using synchrotron microprobes, the authors obtained results indicating that this type of description is incomplete. These field-derived samples gave insight as to the extent and rate of interaction in the environment, atomic scale correlations with other atoms, and allowed them to examine the assumptions currently being used to predict their short and long-term fate and transport. Over these geologically inconsequential but societally important time periods of decades the scientists therefore observed both minimal and substantial momentum in the formation of the equilibrium species as well as found novel species that would have resulted from unpredicted interactions of Pu and U species with other components of their waste streams and surroundings. The objectives of this study were to exploit the access to samples from so many sites and sources to explore the range of species and correlations displayed by U and Pu contamination including the identification of novel types; determine the extent to which these data signified their origins and history; and to elucidate whether and under what conditions these species might have been transformed by chemical reactions after their release.
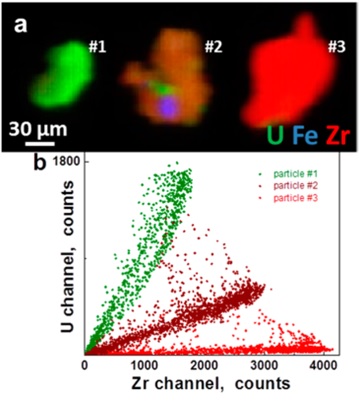
Materials from Chernobyl were exposed to high temperatures that promoted reactions and mixing between the UO2 and the Zr, air, water, graphite, and other materials. The U particles collected from Chernobyl exhibit homogeneous (U, Zr)Ox solid solutions (16−18 wt % Zr) on the μm length scales according to the XRF mapping analyses (Figure 1). This would result from the high temperature promoting the combination of the fuel and cladding materials to give (U, Zr)Ox with varying U:Zr (up to 52 wt % Zr) ratios with some stainless steel inclusions. On the Å length scale however, the EXAFS shows a different result. Zr and U EXAFS can be fit with, respectively, only Zr neighbors at 3.67 Å and only U neighbors at 3.87 Å, implying significant inhomogeneity. This clustering of cations on the nanometer scale is typical of mixed U-Zr oxides. The separate components of the reactor materials, as well as variations in the temperature attained by individual particles, are therefore manifested in the distributions of the elements composing the particles. It is important to note that none of the spectral signatures of U(VI) are observed in these particles, which for EXAFS typically constrains the amount that could be present to less than 10%. Insofar as the initial step in U paragenesis is oxidation so that subsequent alteration phases are uranyl compounds, the absence of U(VI) may be because of the high temperature and subsequently because further oxidation from weathering may have been inhibited by the Zr or a surface modification involving, for example, carbon.
In contrast to the Chernobyl materials, disposal of Pu and U in waste streams sufficiently acidic to react with, at a disposal site at Los Alamos, appears to have promoted both unusual speciation and morphology. The Pu particles found in the XRF maps of soil collected from one hot spot at the Los Alamos TA-21 site that most likely originated as R&D waste, exhibit association with Fe in all three possible ways; typical surface complexes or precipitates on Fe- based mineral phases, isolated Pu particles with proximity but no contact, and single particles composed of Fe−Pu mixtures (Figure 2). The authors interpret these results to suggest that the variation in particle composition is due to reaction of the Pu with chemically diverse soil materials when the putatively acidic solution was neutralized by the soil components.
Since the maps from all of these samples never show any signal in the areas between the larger particles, colloidal or otherwise diffuse Pu must be negligible. That the preponderance of the Pu occurs in μm-scale particles that were most likely not its original form implies that Pu was mobilized and subsequently reacted, nucleating on the crystal surfaces as a surface complex followed by the accumulation of additional Pu on this surface complex to give the bulk species observed here. The EXAFS of the Los Alamos non-Fe-associated Pu particles (Figure 2c) interestingly show only a single O shell at 2.31 Å. The Pu species in these samples are therefore mononuclear and not PuO2-like compounds, analogous to the non-UO2 U(IV) species that have been reported elsewhere1. This poses the question of whether this analogy in species extends to the biogenic origin of these Pu particles as well.
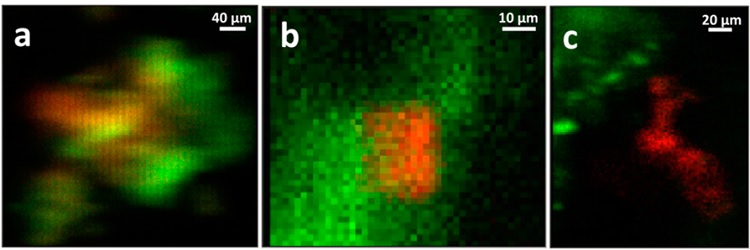
Figure 2. Two-color μ-XRF maps (Pu = red, Fe = green) of Los Alamos TA-21 Pu particles. (a), a mixed Pu−Fe particle, one of two found in the measured samples. (b) Pu particle on an Fe-based mineral grain, one of six similar particles. (c) separated, noncorrelated Pu and Fe particles, one of three similar particles.
These results clearly identify both expected and novel aspects of the environmental chemistry of U and Pu beyond those previously reported from laboratory and mineral studies. One important finding is that the samples from none of these sites showed fluorescence from U and Pu above the detection limit between the particles; these elements were always concentrated in particles without any sign of a more diffuse population. Free U and Pu are likely present for many of the chemical transformations observed, but their concentrations would be low, corresponding with the decades time scales of these reactions. On the Å-scale of speciation, in some samples, particles that were within mm of each other for decades that most likely began as homogeneous species or originated in the same process, exhibit different speciation with no indication of convergence to their presumed thermodynamic minima. This implies that the observed species are the source terms that were inhibited from oxidation. In other samples, Pu is present in unusual, non-PuO2-like species or occur with additional elements whose incorporation would have resulted from transitory local conditions and chemical activation.
Most importantly, they not only provide the molecular scale chemical speciation information that was incomplete in prior reports but also suggest the possible source terms and subsequent history after release. Although these time periods are virtually instantaneous on the geological scale, they are significant on the regulatory one. These results demonstrate that duplicating and predicting the behavior of U and Pu at a specific site is likely to require experiments that duplicate both the site conditions and the chemical form of the contaminant when first released to promote the formation of unexpected or even unknown species that may be present.
D. S. Alessi, J. S. Lezama-Pacheco, J. E. Stubbs, M. Janousch, J. R. Bargar, P. Persson and R. Bernier-Latmani, "The Product of Microbial Uranium Reduction Includes Multiple Species with U(IV)-phosphate Coordination", Geochim. Cosmochim. Acta 131, 115 (2014), DOI: 10.1016/j.gca.2014.01.005.
O. N. Batuk, S. D. Conradson, O. N. Aleksandrova, H. Boukhalfa, B. E. Burakov, D. L. Clark, K. R. Czerwinski, A. R. Felmy, J. S. Lezama-Pacheco, S. N. Kalmykov, D. A. Moore, B. F. Myasoedov, D. T. Reed, D. D. Reilly, R. C. Roback, I. E. Vlasova, S. W. Webb and M. P. Wilkerson, "Multiscale Speciation of U and Pu at Chernobyl, Hanford, Los Alamos, McGuire AFB, Mayak, and Rocky Flats", Environ. Sci. Technol. 49, 6474 (2015), DOI: 10.1021/es506145b.




