A major goal in molecular design and engineering is the creation of new enzymes from scratch. However, the design of 3-dimensional protein architectures from first principles represents a formidable challenge, and as a result, the generation of new enzymatic activities has primarily relied on repurposing the interiors of pre-existing protein folds such as TIM barrels and αβ-hydrolases. In order to circumvent this limitation, Song and Tezcan devised an alternative route by constructing catalytic sites in newly formed, metal-nucleated interfaces between natively monomeric proteins. The resulting supramolecular protein assemblies with interfacial Zn sites are active for ampicillin hydrolysis in vitro as well as inside E. coli cells, allowing these organisms to survive β-lactam antibiotic treatment. Given that a) the monomeric proteins used in this study and their assemblies are structurally and functionally unrelated to natural β-lactamases and b) they represent the first examples of designed protein assemblies with in vivo activities, the findings have important implications for protein evolution and synthetic biology.

Figure 1. Structure-guided design of zinc-complexed AB3 teramer (Zn8:AB34)
To transform the monomeric protein (cytochrome cb562 or cyt cb562) into a tetrameric assembly that can exhibit hydrolytic activity, two distinct Zn-binding motifs were constructed on the surface of a cytochrome cb562 variant (C96RIDC1), whose structure was previously determined in SSRL (PDB 3IQ6).1 One of the Zn binding motifs ensured the stable self-assembly of the tetramer, whereas the other motif anchored coordinatively unsaturated Zn sites for catalysis. Four different variants (AB1-AB4) were designed and structurally characterized by single crystal x-ray diffraction. The x-ray crystal structure of the variant that displays the most desirable biophysical/chemical properties (AB3) was determined at 2.5 Å resolution, using data collected at Beam Line 9-2 at SSRL (Figure 1, PDB 4U9D). The structure of the tetrameric AB3 assembly was consistent with the designed model and formed the basis for subsequent optimization of hydrolytic activity by rational design and directed evolution.
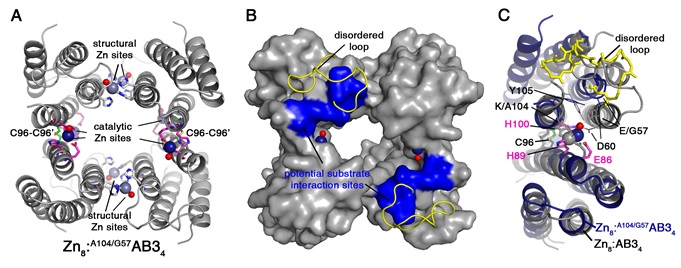
Figure 2. Crystal structure of Zn8:A104/G57AB34. Catalytic and structural Zn ions and the coordinating residues are shown as navy and grey spheres, respectively. The Zn-bound water molecules are shown as red spheres. (B) Surface representation of Zn8:A104/G57AB34. Structurally disordered loops are displayed as yellow ribbons. Potential substrate interaction sites consisting of residues 58-60 and 104-105 are colored in blue. (C) Close-up view of the catalytic Zn sites of Zn8:A104/G57AB34 (navy) overlaid with Zn8:AB34 (grey) structure. Zn-coordinating residues (Glu86, His89, and His100) and the mobile loop are shown as pink and yellow sticks, respectively.
First, a Lys residue (K104), which was found to block substrate access to the catalytic sites, was removed by the K104A mutation. The resulting catalytically active variant, A104AB3, was then demonstrated to properly assemble into a Zn-containing tetramer, Zn8:A104/G57AB34, in the periplasm of E. coli cells and possess ampicillin-hydrolysis activity. This in vivo activity allowed directed evolution studies, in which the residues surrounding the catalytic Zn sites were randomized and the β-lactamase activities of these resulting libraries were screened by survival in the presence of increasing concentrations of ampicillin. The screening led to the most catalytically active variant, A104/G57AB3, which displayed a catalytic proficiency (kcat/Km/kuncat) of 2,300,000. The 2.8-Å crystal structure of the Zn-complex of this variant Zn8:A104/G57AB34 was determined using diffraction data collected at Beam Line14-1 (Figure 2, PDB 4U9E). The structure not only showed that the two mutations (K104A/E57G) caused a significant opening of the tetrameric assembly compared to that of the parent AB3 variant, but they also rendered a normally ordered loop near the catalytic Zn sites highly flexible. Such highly mobile loops, which emerged from in-vivo screening studies in an unplanned fashion, are conserved features of the natural β-lactamases.
J. D. Brodin, A. Medina-Morales, T. Ni, E. N. Salgado, X. I. Ambroggio and F. A. Tezcan, "Evolution of Metal Selectivity in Templated Protein Interfaces", J. Am. Chem. Soc. 132, 8610 (2010) doi: 10.1021/ja910844n
W. J. Song and F. A. Tezcan, "A Designed Supramolecular Protein Assembly with In Vivo Enzymatic Activity", Science 346, 1525 (2014), DOI: 10.1126/science.1259680.




