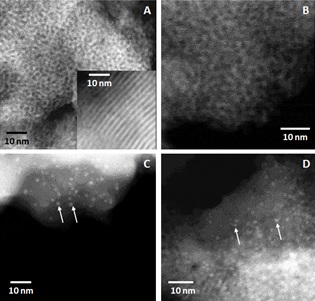
Figure 1. Transmission electron microscopy images of (A) washed, unreacted mesoporous silica (insert: cross section of a particle exhibiting the regular channel array structure), and mesoporous silica reacted with (B) 10 µM Sr, (C) 10 µM U at pH 4.0 with no carbonate and no calcium present, and (D) 10 µM U at pH 9.8 with no carbonate and no calcium present. The white arrows in panels C and D point towards some of the precipitates.
Important reactive phenomena that affect the transport and fate of radioactive material such as uranium (U) and strontium (Sr) in the environment occur at the mineral-water interface, particularly in mesoporous materials which are ubiquitous in surface and near-surface environments, and typically dominate the reactive surface area of geologic media (Singer et al., 2013). Ion sorption and physical bonding forces (including electrostatic forces) can be significantly modified within these confined pore spaces, leading to preferential enrichment of trace elements in mesopores. Pore space confinement may also lead to kinetic restraints on thermodynamically favorable sorption/desorption, precipitation/dissolution, and redox reactions, due to slow migration of metals out of mesopores, chemical gradients within the pore space, or steric constraints for inward migration of larger molecules.
In a recent study, researchers at Lawrence Berkeley National Laboratory investigated ion diffusion into confined pore spaces using a combination of benchtop sorption experiments, TEM, and synchrotron-based x-ray absorption spectroscopy, U(VI) and Sr(II) uptake on mesoporous silica (MCM-41) with a 4.67 nm pore diameter was measured in batch conditions at pH 4.0 and 9.8 as a function of time and metal speciation. Uptake of U was determined for U-hydrolysis, U-CO3, and U-CO3-Ca aqueous species. This suite of techniques enabled determination of the rate of metal sorption and precipitation in the pore spaces, and identification of the reaction products. Their results indicated that Sr and U rapidly diffused into MCM-41. U at higher concentration than 10 µM also rapidly diffuses in, but the higher pore volume U concentration eventually leads to polymerization and precipitation of nano-U-bearing phases (Figures 1 and 2). The steady-state U sorption maximum after 48 hours of exposure to MCM-41 prior to precipitation was dependent on the size and charge of the dominant U species in solution. Precipitation of a U-bearing phase within the silica pore spaces occurred only after a threshold time point and indicated that U uptake was both thermodynamically and kinetically controlled.
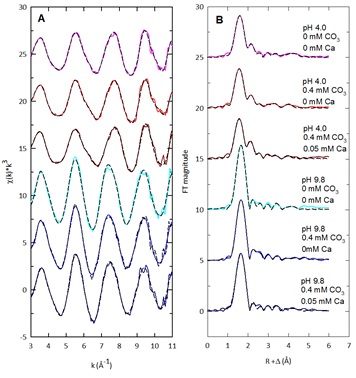
Figure 2. U L3-edge EXAFS spectra (A) and Fourier transform plots (B) of the U sorption samples as a function of solution composition (pH 4.0; red lines, and pH 9.8; blue lines); fits are shown as the black dashed lines.
Initial diffusion and adsorption were controlled by aqueous speciation and precipitation was controlled by the buildup of sorption species that subsequently created a bottleneck effect near pore openings (Figure 3). Acidic solutions were more efficient at extracting U than carbonate solutions once the U has diffused into the mesopore region, and this may explain frequent observations of this behavior in extractions of natural sediments. A nitric acid wash was not completely effective at desorbing U and Sr from the mesoporous silica. The results show that U and Sr diffusion and sorption into mesopores results in a recalcitrant pool of ions that are sequestered in deep internal pore spaces, and this may have significant impacts on attempts to clean up contaminated soils and sediments.
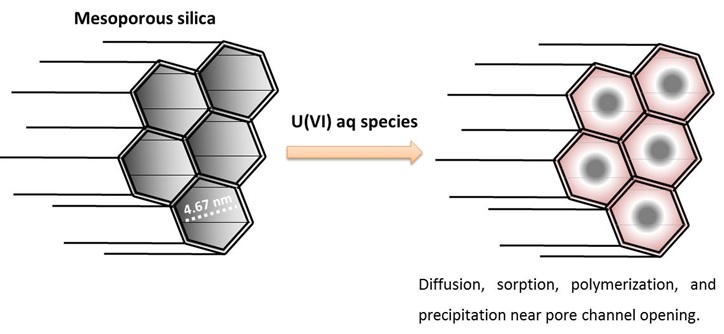
Figure 3. Schematic of uranium interacting with mesoporous silica. As U diffuses into the pore channels, sorption along the pore walls would lead to polymerization and prevent U from diffusing more deeply into the channels. As the concentration gradient near the channel openings increases, polymerization at these corner sites would lead to a precipitation front that moves deeper into the channels along the pore walls through the polymerized surface species.
D. Singer, P. M. Fox, H. Guo, M. A. Marcus and J. A. Davis, "Sorption and Redox Reactions of As(III) and As(V) within Secondary Mineral Coatings on Aquifer Sediment Grains", Environ. Sci. Technol. 47, 11569 (2013), DOI: 10.1021/es402754f
D. M. Singer, H. Guo and J. A. Davis, "U(VI) and Sr(II) Batch Sorption and Diffusion Kinetics into Mesoporous Silica (MCM-41)", Chem. Geol. 390, 152 (2014), DOI: 10.1016/j.chemgeo.2014.10.027




