- Home
- Research program
- Research highlights
- Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers
- Soil organic matter controls Pb release during redox cycles in floodplain soils
- Spatial and Compositional Heterogeneities Control Zn Retention Mechanisms in a Simulated Aquifer
- Calcium-Uranyl-Carbonato Species Kinetically Limit U(VI) Reduction by Fe(II) and Result in U(V)-bearing Ferrihydrite
- Diverse Ammonia-Oxidizing Archaea Dominate Subsurface Nitrifying Communities in Semi-Arid Floodplains
- A Simplified Way to Predict the Function of Microbial Communities
- Complexation Organic Matter Controls Uranium Mobility Anoxic Sediments
- FES-Nanoclusters can mobilize Fe and S from sediment to the groundwater
- Hexavalent uranium storage mechanisms in wet-dry cycled sediments at contaminated DOE sites in the Western U.S.
- Redox-Interfaces can Produce Toxic Arsenic Levels Groundwater...
- Sorption to Organic Matter Controls Uranium Mobility
- Thermodynamic preservation of carbon in anoxic environments
- Iron and sulfur cycling in NRZs controlled by sediment textural and hydrology
- A regional model for uranium redox and mobility
- Long-Term in Situ Oxidation of Biogenic Uraninite in an Alluvial Aquifer: Impact of Dissolved Oxygen and Calcium
- U Release from NRZ sediments is inhibited by Transport and Geochemistry
- Team
- Previous research
- Publications
What is the chemical and physical form of uranium in reduced aquifers?
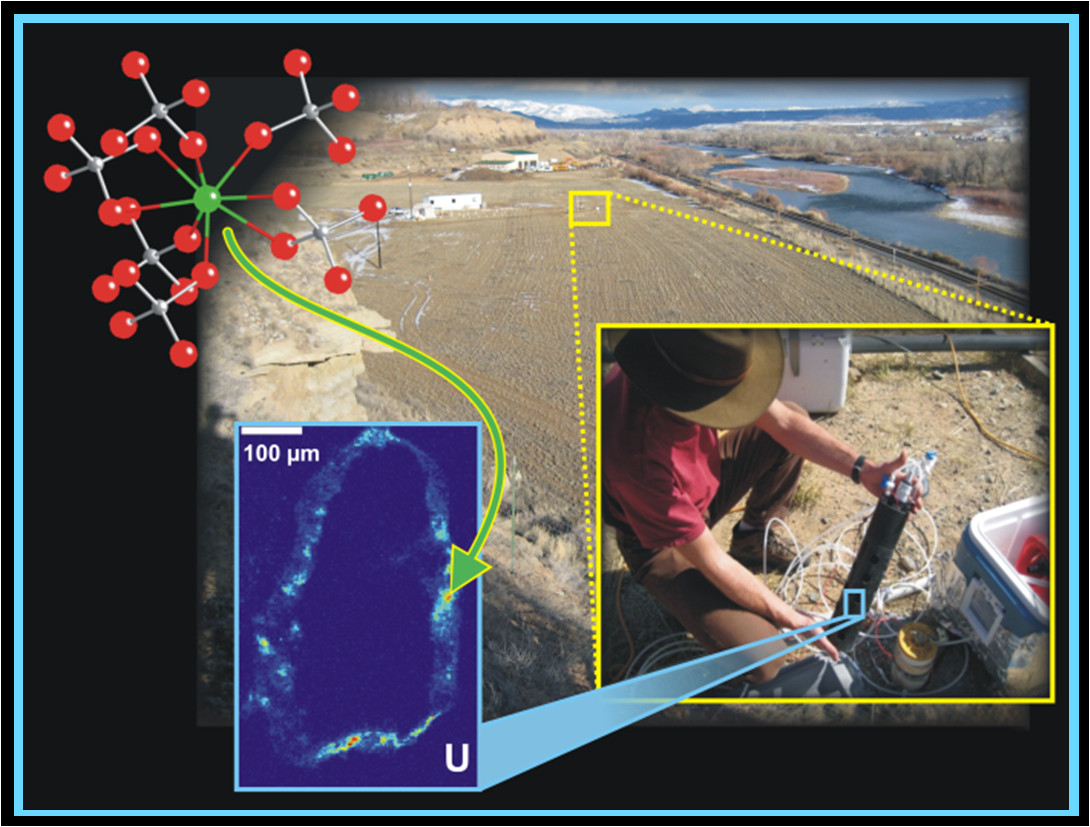
Uranium behavior in the Rifle, CO, aquifer. In order to directly interrogate the chemical and physical form of reduced uranium (U(IV)) in bioremediated sediments within the contaminated aquifer at the Rifle site, a novel technique was developed based on reactors installed in wells (center right). U(IV) was found to be bound to biomass (structural model shown in upper left-hand) within thin (microns) sulfide-rich coatings on mineral grains (bottom left).
Uranium in its oxidized (U(VI)) form, is one of the most common, abundant, and problematic subsurface contaminants at legacy nuclear sites. In contrast, the tetravalent form of uranium (U(IV) ) is relatively insoluble and thus immobile. There is great interest in developing technologies whereby U(VI) can be reduced by bacteria to U(IV) in situ in aquifers as a strategy to bioremediate contaminated groundwater. This can be accomplished by feeding subsurface bacteria with soluble organic carbon, such as acetate (vinegar). The SLAC SFA team has collaborated with the LBNL SFA to study the products of uranium formed during bioremediation of the contaminated aquifer at the Old Rifle, CO site, in Rifle, CO (see associated figure). This location provides an accessible and easily instrumented geochemical proxy for other contaminated aquifers in the western U.S. We have used synchrotron-based x-ray absorption spectroscopy (XAS) and x-ray microscopy (XRM) analyses in conjunction with electron microscopy and aqueous geochemical measurements to show that U(IV) dominantly is bound to bacterial biomass in the aquifer (Bargar et al., Proc. Natl. Acad. Sci. U.S.A., 2013, 110(12): 4506-4511). Moreover, this work has uncovered evidence for a novel abiotic-biotic reaction mechanism. These results have major implications for uranium stability in bioremediated aquifers, indeed for bioremediation strategies in general, and suggest that revision of biogeochemical reactive transport models is needed. For more information, visit:http://www-ssrl.slac.stanford.edu/content/science/highlight/2013-03-31/b....
A large fraction of sediment-bound uranium at the Rifle site occurs within organic-rich lenses of sediment. Slow release of this uranium pool to the aquifer is believed to be an important mechanism maintaining the persistent groundwater urainum plume at this site. This type of mechanism may be important at other uranium-contaminated sites in the Colorado River basin where uranium groundwater plumes have proven to be longer-lived than expected.The behavior of uranium, sulfur, and iron in these sediments is intimately connected to the sediment organic carbon and the microbial metabolism that shuttles electrons from carbon to these species. We are investigating the molecular-scale speciation of organic carbon, iron, uranium, and sulfur in the sediments using synchrotron x-ray based techniques and the mechanisms of uranium reduction in these sediments. We are also studying the mechanisms and rates of uranium oxidation and release from the reduced organic-rich sediments. This work is help to inform biogeochemical reactive transport models to describe uranium release to the aquifer and the mechanistic linkages between organic carbon, iron, uranium, and sulfur biogeochemical cycles.
What controls the speciation of bacterially reduced uranium?

Noncrystalline U(IV) bound to EPS. STXM images showing uranium (top image) and EPS (bottom) maps around a clump of Shewanella oneidensis MR-1 cells. The tricolor map shows three main biological polymers: protein, lipids, and polysaccharides. Bacterial cells, rich in proteins with lipid membranes, appear as elongated red structures. In contrast, EPS contains abundant polysaccharides and appears as blue biomass. Comparison of the top and bottom images shows that U(IV) is mostly associated with EPS.
One of the major unresolved questions in uranium redox biogeochemistry is, What is the biogeochemical “switch” that leads to the production of either uraninite (UO2) or non-crystalline forms of U(IV)? Both chemical forms of uranium can be produced by bacteria when they reduce soluble U(VI) to U(IV) (Bernier-Latmani et al., Environ. Sci. Technol. 2010, 44(24): 9456-9462; Alessi et al., Environ. Sci. Technol. 2012, 46(11): 6150-6157). However, they are quite different in terms of their reactivity. Uraninite is generally considered to be the most desirable product for bioremediation applications because it is believed to be more stable than noncrystalline U(IV) in the presence of oxidants such as oxygen, nitrate or nitrite. If we understood the fundamental biogeochemical controls over the type of U(IV) produced, then we could use this knowledge to improve the effectiveness of bioremediation strategies.
A SLAC SFA project led by Rizlan Bernier-Latmani at EPFL (Lausanne, Switzerland) used advanced synchrotron techniques to study this problem (Stylo et al., Environ. Sci. Technol. 2013, 47(21):12351−12358). Scanning transmission X-ray microscopy (STXM) was used to map the distributions of bacterial cells and uranium products at ~ 40 nm resolution. We used X-ray spectroscopy (XAS) to determine the molecular structure around uranium and thus to learn if it occurred as uraninite or noncrystalline U(IV).
STXM measurements showed that noncrystalline U(IV) was associated with extracellular polymeric substances (EPS). EPS is produced by bacteria to adhere to surfaces and construct biofilms, which provide a more controlled environment for microorganisms than bulk solution. EPS contains macromolecules such as polysaccharides, proteins, lipids and DNA that contain strong binding sites for U(IV). Noncrystalline U(IV) and EPS were produced when oxyanions commonly found in groundwater (sulfate, silicate and phosphate) were present. However, if these anions were absent, then little EPS was produced, and uraninite was the dominant product. These results show that the type of U(IV) produced depends on the interplay between groundwater chemistry and microbial physiology. This conclusion is a major step forward and suggests a major role for EPS in uranium biogeochemistry.
What controls the stability of organic carbon in Artic soils?

Importance of iron to Arctic soil biogeochemistry. The golden color of the Arctic soil water pictured in this figure (in the glass jar) derives from naturally abundant iron(III)-organic matter complexes. Binding of iron to organic matter increases the bioavailability of iron to bacterial metabolism and modifies the stability of organic carbon. Location: Barrow Environmental Observatory, Barrow, Alaska. Pictured (left to right): Elizabeth Herndon (ORNL) and John Bargar (SLAC SFA research manager and co-principal investigator).
Metal redox cycles help to mediate NOM degradation and climate‐land interactions in the Arctic. For example, dissolved iron in Arctic coastal plain soil water supports iron reducing microbial metabolism and simultaneously suppresses methanogenesis. Thus, iron biogeochemistry helps to regulate both the type and extent of tundra greenhouse gas emissions. Little is known about these processes at the molecular scale. Yet, such knowledge is needed to improve process representation at the sub‐grid scale for improved accuracy of climate‐land model predictions.
We undertook a feasibility study to use X-ray absorption spectroscopy (XAS) to examine the speciation of soil organic carbon and their interactions with metals in Arctic soils from the Barrow, Alaska Environmental Observatory. This work was performed in collaboration with the NGEE–Arctic science team and in particular with the ORNL biogeochemistry group. Carbon and nitrogen K-edge XAS measurements provide valuable information about the functional group chemistry of soil organic matter, whereas iron K-edge XAS measurements provide information about the local molecular structure around iron in soluble iron-organic matter complexes, abundant in Arctic soil organic matter. Suspended iron-DOM complexes are believed to profoundly impact microbial degradation of natural organic matter. These investigations will contribute to new process models and insights into biogeochemical dynamics controlling carbon cycling and greenhouse gas emissions in Arctic ecosystems. This study is not being continued under the current SFA program.
What is the structure and reactivity of ferrihydrite?
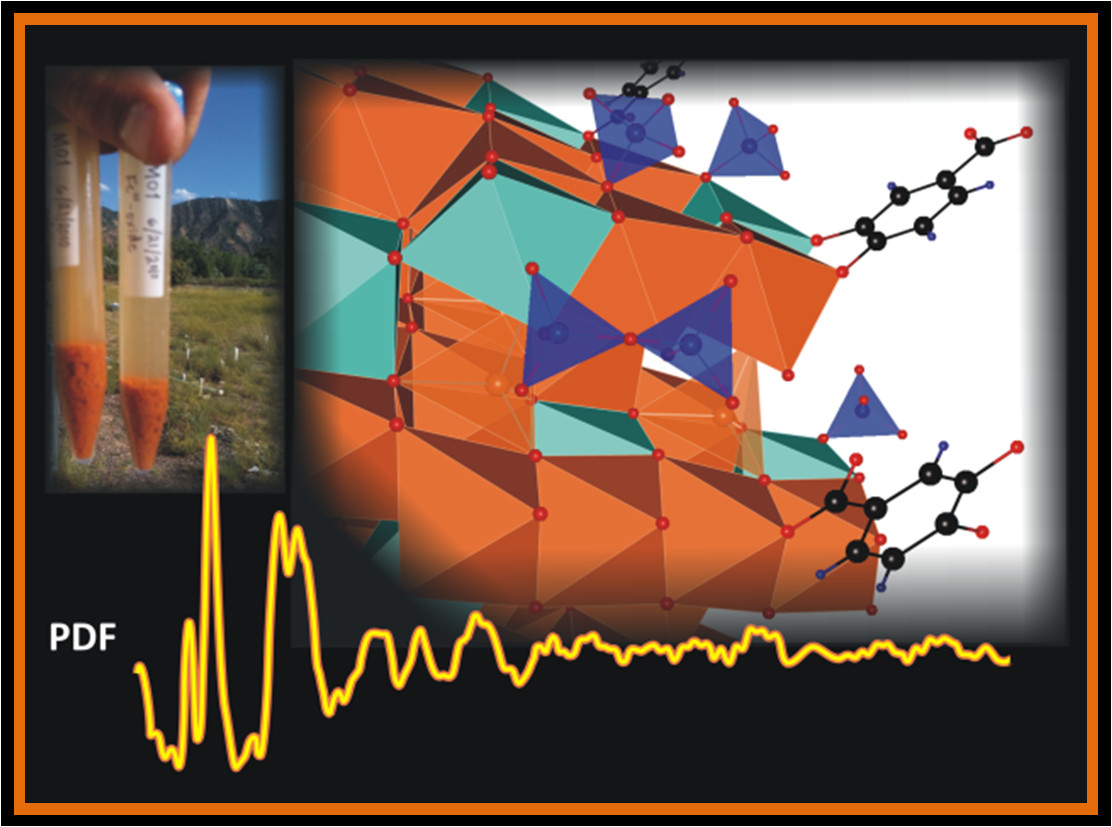
Molecular-scale structure of ferrihydrite. Ferrihydrite is common in aquifers and soils, such as at the Rifle, CO, aquifer (upper left). X-PDF data (bottom) provides information on the structure (above center) and particle size of this abundant, reactive poorly-crystalline nano-material.
Iron oxides are among the most important reactive solids in subsurface environments, acting as natural filters and as oxidants/reductants of inorganic contaminants. Ferrihydrite, a nanoscale hydrated Iron(III) oxide, is of particular importance because it is continuously produced in surface and ground waters forming grain coatings that mask the identity of the underlying sediment. Ferrihydrite is metastable and transforms to more stable iron oxides such as goethite (α-FeOOH) and hematite (α-Fe2O3). If reducing conditions are present, such as may occur in an aquifer during uranium bioremediation, ferrihydrite may transform into iron(II)-bearing minerals such as magnetite (Fe3O4) as well as goethite and other iron(III) bearing minerals. Uranium sorbed onto ferrihydrite can be remobilized into the aquifer or immobilized within iron oxides. Because of the abundance and ongoing formation/transformation of iron oxides in the subsurface, these processes have the potential to naturally remediate substantial amounts of subsurface uranium contamination. Moreover, because ferrihydrite is readily accessible to bacteria, chemically and physically, it plays a major role in supporting iron-reducing bacteria. These bacteria are enormously important to metal reduction in aquifers and carbon cycling in Arctic soils.
We have used synchrotron high-energy x-ray scattering coupled with pair distribution function analysis (X-PDF) to study the structure, composition, and magnetic behavior of ferrihydrite (see associated figure). This work has revealed that disordered ferrihydrite exhibits significant structural defects on the form of Fe3+ vacancies as well as size-dependent lattice strain (Michel et al. Proc. Natl. Acad. Sci. U.S.A. 2010, 107(7): 2787-2792). Ferrihydrite structural defects increase and particle size decreases with increasing incorporation of Si, Al and/or organic matter (Cismasu et al., Geochim. Cosmochim. Acta 2013, 92: 275-291; Geochim. Cosmochim. Acta 2013, 119: 46-60). This work contributes to the fundamental scientific basis for understanding the reactivity of this important natural material in soils and aquifers.
What is the structure and stability of biogenic uraninite?
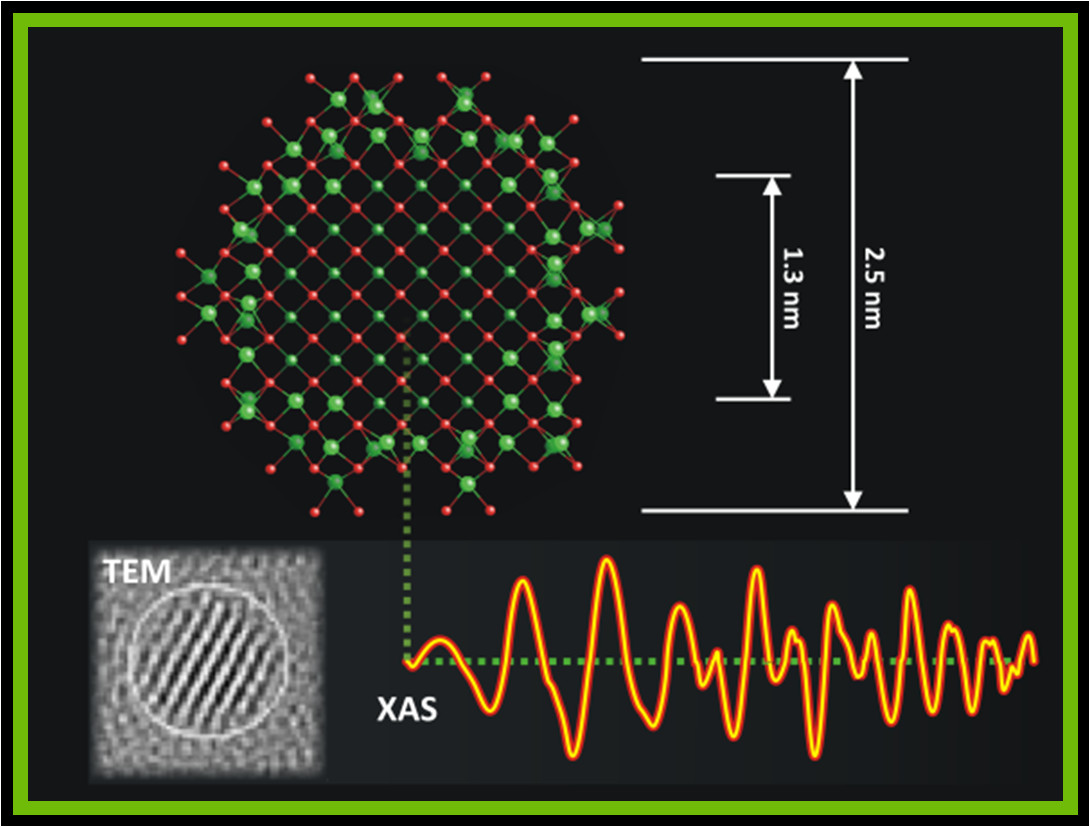
Molecular-scale structure of biogenic uraninite. Uraninite produced by bacteria occurs as ~3 nm-diameter nanoparticles. X-ray absorption spectra (data, lower right) show that biogehnic uraninite particles have highly ordered cores surrounded by a disordered outer layer. The large degree of order in the particles helps to explain the reactivity of this material.
Uranium in its oxidized (U(VI)) form, is one of the most common, abundant, and problematic subsurface contaminants at legacy nuclear sites. In contrast, the tetravalent form of uranium (U(IV) ) is relatively insoluble and thus immobile. There is great interest in developing technologies whereby U(VI) can be reduced by bacteria to U(IV) in situ in aquifers as a strategy to bioremediate contaminated groundwater. Macrocrystalline uraninite (nominal formula UO2) is one of the most thermodynamically stable forms of U(IV) and therefore is a desirable reduction product. Biogenic uraninite is nanoparticulate with a typical particle size of < 3 nm. Because of its diminutive size, there is concern that it may have extensive lattice strain and disorder, which could destabilize the material. Geological uraninite ubiquitously incorporates foreign cation impurities into its structure, including excess O and/or U(VI), inducing substantial deformation of the structure at the molecular scale. Substituted uraninites are stable under conditions where pure stoichiometric UO2.00 corrodes. These considerations raise numerous questions about the structure and composition of biogenic uraninite. For example, does biogenic uraninite contain structural impurities or exhibit nano-size-related strain? How do these factors impact its stability with respect to reaction with natural oxidants and complexing agents such as O2 and carbonate anion?
We have performed series of investigations to answer these questions. We have used synchrotron-based x-ray absorption spectroscopy (XAS) and x-ray diffraction (XRD) to show that the molecular- and nanoscale structures of biogenic uraninites produced by a wide variety of bacteria are, surprisingly, consistent with pure macrocrystalline UO2; there is no evidence for the expected strain of the particle interiors (Sharp et al., Environ. Sci. Technol. 2009, 43(21): 8295-8301). Rather, biogenic uraninite nanoparticles exhibit relatively pure sturctures (relatively little oxygen or U(VI) incorporation) and have highly ordered structures (see figure above; Schofield et al., Environ. Sci. Technol. 2008, 42(21): 7898-7904). However, microbial reduction of U(VI) in the presence of Mn2+ resulted in structural incorporation of Mn2+ in uraninite, smaller particle sizes, and loss of structural order. This Mn-reacted material was less soluble and less susceptible to oxidative dissolution than pure biogenic uraninite (Veeramani et al., Environ. Sci. Technol. 2009, 43(17): 6541–6547). The lack of strain in biogenic nano-uraninite may help to explain the fact that it exhibits similar oxidation and dissolution rates as macrocrystalline uraninite in the laboratory (Ulrich et al., Geochim. Cosmochim. Acta 2009, 73(20): 6065–6083) and in aquifers (Campbell et al., Envir. Sci. Technol. 2011, 45(20): 8748-8754). We have shown that the common groundwater solute Ca2+ interacts strongly with surfaces of uraninite, enhancing its stability (Cerrato et al., Environ. Sci. Technol., 2012, 46(5): 2731-2737) and that biogenic uraninite is more stabile than U(IV) bound to bacterial biomass (Cerrato et al., Environ. Sci. Technol, Environ. Sci. & Technol., 2013, 47 (17): 9756–9763).

