- Home
- Research program
- Research highlights
- Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers
- Soil organic matter controls Pb release during redox cycles in floodplain soils
- Spatial and Compositional Heterogeneities Control Zn Retention Mechanisms in a Simulated Aquifer
- Calcium-Uranyl-Carbonato Species Kinetically Limit U(VI) Reduction by Fe(II) and Result in U(V)-bearing Ferrihydrite
- Diverse Ammonia-Oxidizing Archaea Dominate Subsurface Nitrifying Communities in Semi-Arid Floodplains
- A Simplified Way to Predict the Function of Microbial Communities
- Complexation Organic Matter Controls Uranium Mobility Anoxic Sediments
- FES-Nanoclusters can mobilize Fe and S from sediment to the groundwater
- Hexavalent uranium storage mechanisms in wet-dry cycled sediments at contaminated DOE sites in the Western U.S.
- Redox-Interfaces can Produce Toxic Arsenic Levels Groundwater...
- Sorption to Organic Matter Controls Uranium Mobility
- Thermodynamic preservation of carbon in anoxic environments
- Iron and sulfur cycling in NRZs controlled by sediment textural and hydrology
- A regional model for uranium redox and mobility
- Long-Term in Situ Oxidation of Biogenic Uraninite in an Alluvial Aquifer: Impact of Dissolved Oxygen and Calcium
- U Release from NRZ sediments is inhibited by Transport and Geochemistry
- Team
- Previous research
- Publications
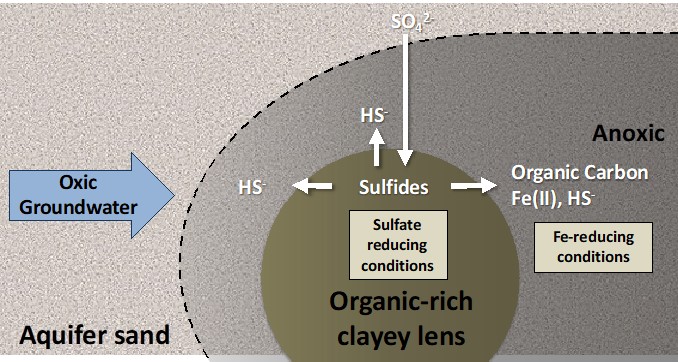 Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers Export of carbon from organic-enriched lenses stimulates reducing conditions in oxic sandy aquifers, promoting redox cycling in high-flow zones. Export of carbon, FeExport of carbon from organic-enriched lenses stimulates reducing conditions in oxic sandy aquifers, promoting redox cycling in high-flow zones.
Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers Export of carbon from organic-enriched lenses stimulates reducing conditions in oxic sandy aquifers, promoting redox cycling in high-flow zones. Export of carbon, FeExport of carbon from organic-enriched lenses stimulates reducing conditions in oxic sandy aquifers, promoting redox cycling in high-flow zones.
The Science
Aquifers contain abundant organic-enriched, fine-grained, and sulfidic lenses. While it is widely understood that these heterogeneities are important sources of organic carbon, Fe(II), and S, the reactive transport mechanisms by which these reactive species influence biogeochemical function in surrounding aquifer environments are poorly understood. Recent numerical studies have modeled the microbially-driven reduction reactions as occurring internal to the sediment lenses. However, in our two experimental studies, we show that in addition to reduced aqueous species (e.g., Fe(II), HS-) organic carbon is exported from organic-enriched lenses and stimulate microbial anaeerobic reduction in the surrounding aquifer. Thus, the sandy aquifer matrix is recruited as a microbial redox-active zone.
The Impact
This finding implies that an additional reactive transport mechanism and much more long-lived pool of reducing equivalents controls redox cycling in oxic aquifers than has been implemented in recent numerical models. Our studies show that microbial redox cycling of micronutrients and contaminants can be sustained within nominally oxic aquifers in the vicinity of organic-enriched sediment lenses. This means that a larger volume of the subsurface matrix is redox active. However, the redox conditions in these “reducing halos” in the surrounding, sandy environment are much more sensitive to influx of oxidants than are the lenses, and, hence, the mobility of redox sensitive micronutrients and contaminants can quickly change within this environment.
Summary
In this study, we used natural floodplain sediments and examined the influence of organic-enriched, fine-grained lenses on redox conditions in surrounding sandy aquifer sediments, and on consequential implications for speciation and mobility of Zn (Engel, 2021) and As (Kumar, 2020). Synchrotron X-ray absorption spectroscopy at SSRL beam lines 4-3 and 7-3 showed that FE (II) minerals, including FeS, and elemental S, were present in the surrounding, nominally oxic, aquifer in abundances that exceeded what abiotic, aqueous reduced products could explain. We conclude that, when sulfate concentrations in the groundwater are high, the export of reducing capacity (“exported reactivity”) from fine-grained, sulfidic lenses into aquifer sand can promote microbial Fe and sulfate reduction that in turn leads to FeS precipitation and elemental S formation. Elemental S can then react with As to form thiolated As species, which appear to have a higher solubility and mobility than other As species. In Contrast, when Zn(II) is present as a dissolved contaminant, it reacts strongly with dissolved HS- and Zn (but not impacting Fe and organic matter export) from the organic-enriched lenses. Thus, the combination of high-sulfate groundwater and heterogeneous sediment composition (e.g., fine-grained, organic-rich/coarse interfaces) can locally promote severely elevated As concentrations, even when sediment As concentrations are below the global average. Conversely, Zn attenuation is amplified by the same sediment heterogeneities
Contact
Kristin Boye, Staff scientist
SLAC National Laboratory
2575 Sand Hill Rd., Menlo Park, CA
Funding
The project was conceived and supported by the SLAC Floodplain Hydro-Biogoechemistry Science Focus Area project funded by the US Department of Energy, Office of Biological and Environmental Research, Earth and Environmental Systems Sciences Division (EESS) under contract no. DEAC0276SF00515SBR (SLAC National Accelerator Laboratory).
The United States-Israel Binational Agricultural Research and Development Fund (BARD; grant no. FI-569-2018) and the Israeli Council for Higher Education provided financial support for Engel through postdoctoral fellowships. Additional support (for Engel and Fendorf) was provided by SBR Project DE-SC0020205. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (contract no. DE-AC0276SF00515).
Publications
M. Engel, et al. “Simulated Aquifer Heterogeneity Leads to Enhanced Attenuation and Multiple Retention Processes of Zinc”. Environ. Sci. Technol. 55, 5, 2939–2948 (2021). [https://dx.doi.org/10.1021/acs.est.0c06750] OSTI link: https://www.osti.gov/pages/biblio/1782085
N. Kumar, et al. “Redox heterogeneities promote thioarsenate formation and release into groundwater from low arsenic sediments.” Environ. Sci. Technol. 54, 6, 3237–3244 (2020). [DOI:10.1021/acs.est.9b06502] OSTI link:https://www.osti.gov/pages/biblio/1633945-redox-heterogeneities-promote- thioarsenate-formation-release-groundwater-from-low-arsenic-sediments
Related Links
None





