- Home
- Research program
- Research highlights
- Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers
- Soil organic matter controls Pb release during redox cycles in floodplain soils
- Spatial and Compositional Heterogeneities Control Zn Retention Mechanisms in a Simulated Aquifer
- Calcium-Uranyl-Carbonato Species Kinetically Limit U(VI) Reduction by Fe(II) and Result in U(V)-bearing Ferrihydrite
- Diverse Ammonia-Oxidizing Archaea Dominate Subsurface Nitrifying Communities in Semi-Arid Floodplains
- A Simplified Way to Predict the Function of Microbial Communities
- Complexation Organic Matter Controls Uranium Mobility Anoxic Sediments
- FES-Nanoclusters can mobilize Fe and S from sediment to the groundwater
- Hexavalent uranium storage mechanisms in wet-dry cycled sediments at contaminated DOE sites in the Western U.S.
- Redox-Interfaces can Produce Toxic Arsenic Levels Groundwater...
- Sorption to Organic Matter Controls Uranium Mobility
- Thermodynamic preservation of carbon in anoxic environments
- Iron and sulfur cycling in NRZs controlled by sediment textural and hydrology
- A regional model for uranium redox and mobility
- Long-Term in Situ Oxidation of Biogenic Uraninite in an Alluvial Aquifer: Impact of Dissolved Oxygen and Calcium
- U Release from NRZ sediments is inhibited by Transport and Geochemistry
- Team
- Previous research
- Publications
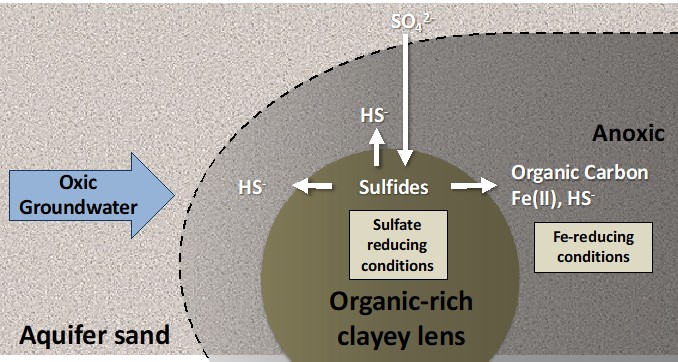 Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers
Exported organic carbon promotes reducing conditions and redox cycling in oxic aquifers
N. Kumar, et al. “Redox heterogeneities promote thioarsenate formation and release into groundwater from low arsenic sediments.” Environ. Sci. Te
chnol. 54, 6, 3237–3244 (2020). [DOI:10.1021/acs.est.9b06502] https://www.osti.gov/pages/biblio/1633945-redox-heterogeneities-promote- thioarsenate-formation-release-groundwater-from-low-arsenic-sediments
The Science: Aquifers contain abundant organic-enriched, fine-grained, and sulfidic lenses. While it is widely understood that these heterogeneities are important sources of organic carbon, Fe(II), and S, the reactive transport mechanisms.... Read more >
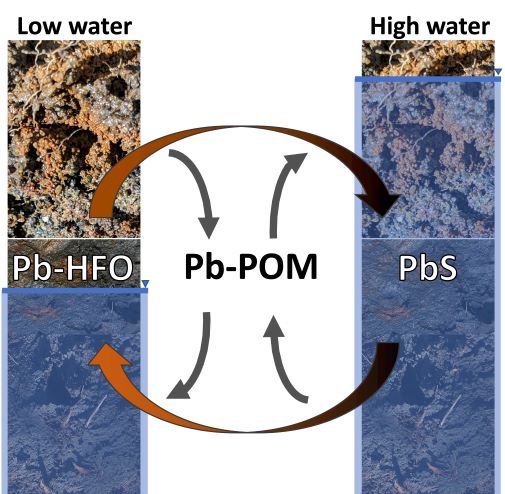 Soil organic matter controls Pb release during redox cycles in floodplain soils
Soil organic matter controls Pb release during redox cycles in floodplain soils
Dewy, C. et al. “Porewater Lead Concentrations Limited by Particulate Organic Matter Coupled with Ephemeral Iron(III) and Sulfide Phases during Redox Cycles within Contaminated Floodplain Sediments.” Environ. Sci. Technol. 9, 5878-5886 (2021). https://dx.doi.org/10.1021/acs.est.0c08162
The Science: Lead contamination in soils is a major threat to water quality. Although Pb tends to occur in sparingly soluble minerals, changes in dissolved oxygen concentrations can promote dissolution of these minerals, potentially causing... Read more >
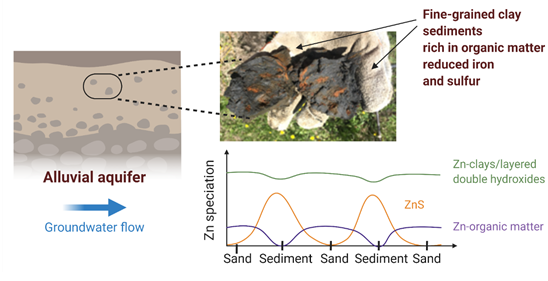 Spatial and Compositional Heterogeneities Control Zn Retention Mechanisms in a Simulated Aquifer
Spatial and Compositional Heterogeneities Control Zn Retention Mechanisms in a Simulated Aquifer
Engel, M., Boye, K., Noël, V., Babey, T., Bargar, J.R., Fendorf, S. (2021) Simulated Aquifer Heterogeneity Leads to Enhanced Attenuation and Multiple Retention Processes of Zinc. Environmental Science & Technology. https://dx.doi.org/10.1021/acs.est.0c06750
The Science: Alluvial aquifers are an essential source of groundwater worldwide, particularly for water storage purposes. Fine-grained lenses of clay and organic matter, enriched in iron and sulfur, are abundant within aquifers and support cycling of... Read more >
 Calcium-Uranyl-Carbonato Species Kinetically Limit U(VI) Reduction by Fe(II) and Result in U(V)-Bearing Ferrihydrite
Calcium-Uranyl-Carbonato Species Kinetically Limit U(VI) Reduction by Fe(II) and Result in U(V)-Bearing Ferrihydrite
Dewey, C., Sokaras, D., Kroll, T., Bargar, J.R., Fendorf, S. Calcium-Uranyl-Carbonato Species Kinetically Limit U(VI) Reduction by Fe(II) and lead to U(V) - Bearing Ferrihydrite. Environmental Science and Technology (Accepted Manuscript).
The Science Uranium (U) solubility determines the dissolved concentrations and thus its threat to water quality. It is therefore essential to understand the controls on U solubility to predict and mitigate the impacts of U contamination. Read more >
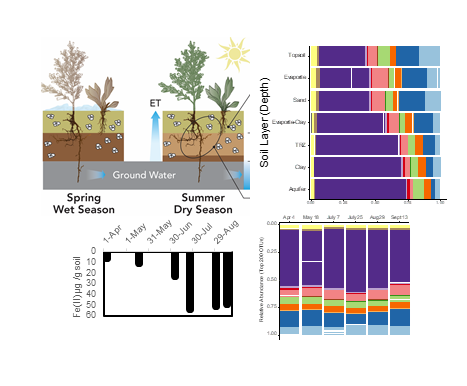 Microbial Communities in Floodplain Soils Remain Unchanged Throughout Seasonal Redox and Water Table Flux
Microbial Communities in Floodplain Soils Remain Unchanged Throughout Seasonal Redox and Water Table Flux
Tolar, B.B., Boye, K., Bobb, C., Maher, K., Bargar, J.R., Francis, C.A. Stability of floodplain subsurface microbial communities through seasonal hydrological and geochemical cycles. Frontiers in Earth Science – Biogeoscience (in revision).
The Science Microbial communities play a crucial role in environmental systems, mediating biogeochemical reactions through metabolic processes that can vary depending on environmental conditions. Read more >
Diverse A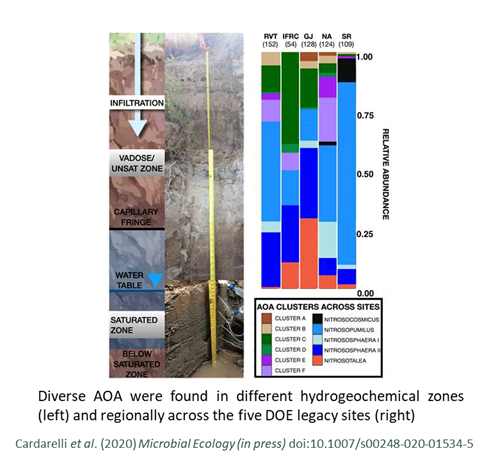 mmonia-Oxidizing Archaea Dominate Subsurface Nitrifying Communities in Semi-Arid Floodplains
mmonia-Oxidizing Archaea Dominate Subsurface Nitrifying Communities in Semi-Arid Floodplains
Cardarelli, E. L., Bargar, J.R., Francis, C.A. Diverse Thaumarchaeota dominate subsurface ammonia-oxidizing communities in semi-arid floodplains in the Western United States. Microbial Ecology (in press)
DOI:10.1007/s00248-020-01534-5
Ammonia oxidation may be driven by archaea rather than bacteria within the riparian subsurface. Read more >
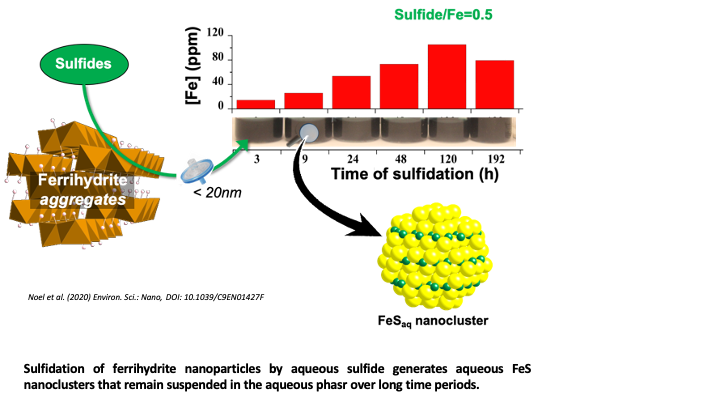 FeS Nanoclusters Can Mobilize Fe and S from sediment to the Groundwater
FeS Nanoclusters Can Mobilize Fe and S from sediment to the Groundwater
Noël, V., Kumar, N., Boye, K., Barragan, L., Lezama-Pacheco, J., Chu, R., Tolic, N., Gordon E. Brown Jr., G.E, Bargar, J.R. (2020) FeS Colloids – Formation and Mobilization Pathways in Natural Waters. Environmental Science Nano., Accepted Manuscript.
DOI: 10.1039/C9EN01427F
Ferrihydrite Sulfidation Promotes FeS Nanocluster Formation. Read more >
Com plexation by Organic Matter COntrols Uranium Mobility in Anoxic Sediments
plexation by Organic Matter COntrols Uranium Mobility in Anoxic Sediments
Bone, S.E.; Cliff, J.; Weaver, K.; Takacs, C.J.; Roycroft, S.; Fendord, S.; Bargar, J.R. Uranium complexation by organic matter and clay minerals in anoxic contaminated sediments. Environmental Science and Technology 2020, 54, 1493-1502.
https://doi.org/10.1021/acs.est.9b04741
Uranium(IV) adsorbs to organic matter in anoxic alluvial sediment, which may be mobilized through desorption and colloidal release. Read more >
 |
Redox Interfaces Can Produce Toxic Arsenic Levels in Groundwater from Low Arsenic-Abundance Sediments
Kumar, N., Noël,V., Planer-Friedrich, B., Besold, J., Lezama-Pacheco, J., Bargar, J.R., Brown, G. E., Fendorf, S. Boye, K Redox Heterogeneities Promote Thioarsenate Formation and Release into Groundwater from Low Arsenic Sediments. Environmental Science & Technology, 54 (2020), 3237-3244;
https://pubs.acs.org/doi/10.1021/acs.est.9b06502
New process observed for uranium accumulation and release at contaminated DOE sites. Read more >
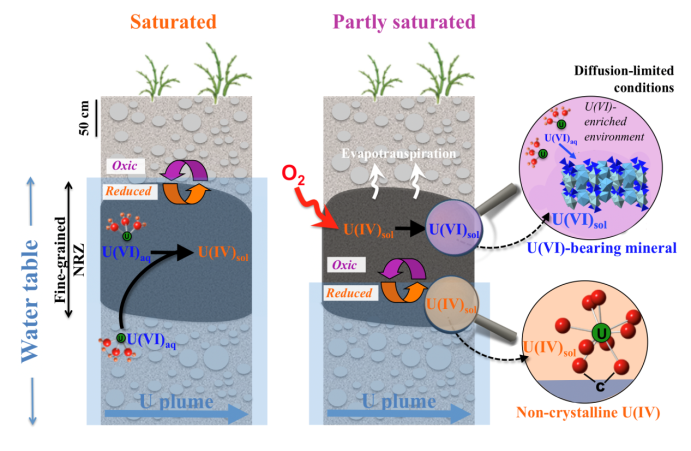 |
Hexavalent uranium storage mechanisms in wet-dry cycled sediments at contaminated DOE sites in the Western U.S
Noël, V.; Boye, K.; Kukkadapu, R. K.; Li, Q.; Bargar, J. R. Uranium storage mechanisms in wet-dry redox cycled sediments. Water Research. Water Research 152 (2019), 251-263;
https://doi.org/10.1016/j.watres.2018.12.040
Sulfate-rich groundwater promote formation of thioarsenates at fine-coarse sediment interfaces and increases arsenic solubility Read more >
 |
A Simplified Way to Predict the Function of Microbial Communities
K. Boye, A.H. Hermann, M.V. Schaefer, M.M. Tfaily, and S. Fendorf, “Discerning microbially mediated processes during redox transitions in flooded soils using carbon and energy balances.” Frontiers in Environmental Science (2018) [DOI: 10.3389/fenvs.2018.00015]
https://www.frontiersin.org/articles/10.3389/fenvs.2018.00015/full
This pioneering study offers an easier approach to study how microbes work and could help scientists advance models of the cycling of elements and nutrients in frequently flooded soils. Read more >
 |
Sorption to Organic Matter Controls Uranium Mobility
Bone SE, Dynes JJ, Cliff J, & Bargar JR “Uranium(IV) adsorption by natural organic matter in sediments.” Proceedings of the National Academy of Sciences of the United States of America 114(4), 711-716. [10.1073/pnas.1611918114]
http://www.pnas.org/content/114/4/711.abstract
A new multi-technique study using X-ray absorption spectroscopy at SSRL, nanoscale secondary Ion mass spectroscopy at EMSL, and scanning transmission X-ray microscopy at the CLS has revealed crisp new details about the mechanisms of uranium binding in sediments. Surfaces of natural organic matter bind uranium more strongly than minerals under field-relevant conditions. Read more >
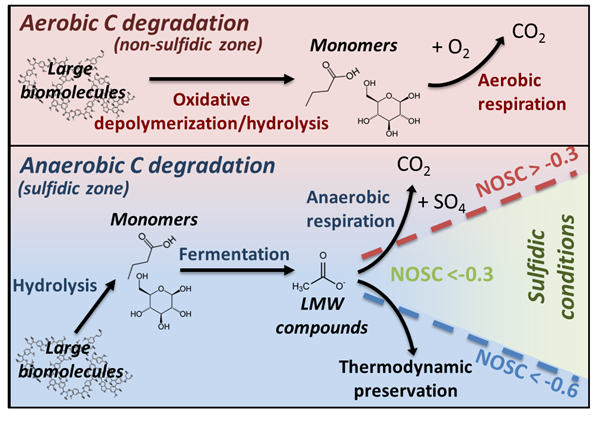 |
Thermodynamic preservation of carbon in anoxic environments
Boye, K., Noël, V., Tfaily, M.M., Bone, S.E., Williams, K.H., Bargar, J.R., Fendorf, S. (2017) Thermodynamically controlled preservation of organic carbon in floodplains. Nature Geoscience [10.1038/ngeo2940]
http://www.nature.com/ngeo/journal/vaop/ncurrent/full/ngeo2940.html
A new study combining X-ray absorption spectroscopy (XAS) at SSRL with Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) at EMSL provides new insights on why carbon persists in waterlogged soil and subsurface sediments . Energetic constraints prevent microbial respiration of certain organic carbon compounds, leaving a pool of water-soluble carbon that is susceptible to oxidation or export and subsequent decomposition in downstream, aerated environments. Read more >
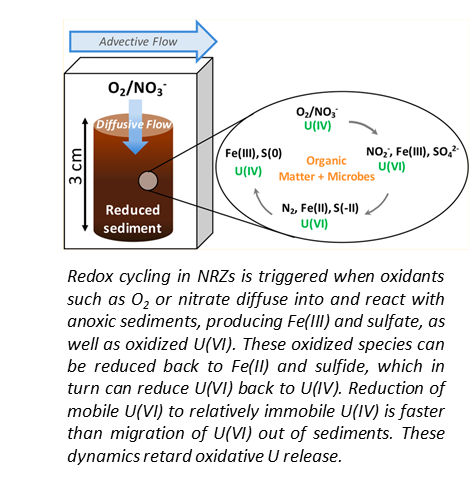 U Release from NRZ sediments is Inhibited by Transport and Geochemistry
U Release from NRZ sediments is Inhibited by Transport and Geochemistry
Bone, S.E.; Cahill, M.R.; Jones, M.E.; Fendorf, S.; Davis, J.A.; Williams, K.H.; Bargar, J.R. Oxidative Uranium Release from Anoxic Sediments under Diffusion-Limited Conditions. Environmental Science and Technology 2017, 51, 11039−11047.
https://pubs.acs.org/doi/10.1021/acs.est.7b02241
The Science
Floodplain aquifers undergo seasonal water table fluctuations that drive redox cycling of subsurface species, including contaminants such as uranium. We posited that seasonal intrusion of O2 into anoxic sediments will stimulate production of NO3- followed by denitrification. Read more >
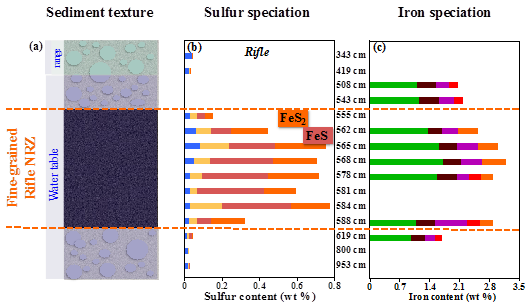 Iron and sulfur cycling in NRZs controlled by sediment textural and hydrology
Iron and sulfur cycling in NRZs controlled by sediment textural and hydrology
Noël V, Boye K, Kukkadapu RK, Bone SE, Lezama Pacheco JS, Cardarelli E, Janot N, Fendorf S, Williams KH, Bargar JR (2017) Understanding controls on redox processes in floodplain sediments of the Upper Colorado River Basin. Science of The Total Environment,
https://doi.org/10.1016/j.scitotenv.2017.01.109
Detailed characterization of the molecular structures of Fe and S, on depth-resolved field samples from floodplain sediments, were performed using x-ray absorption spectroscopy (XAS) and X-ray microspectroscopy at SSRL combined with mössbauer spectroscopy at EMSL. The results revealed that organic carbon content, moisture, and particle size control the distribution and reactivity of redox constraints in the floodplain subsurface. Read more >
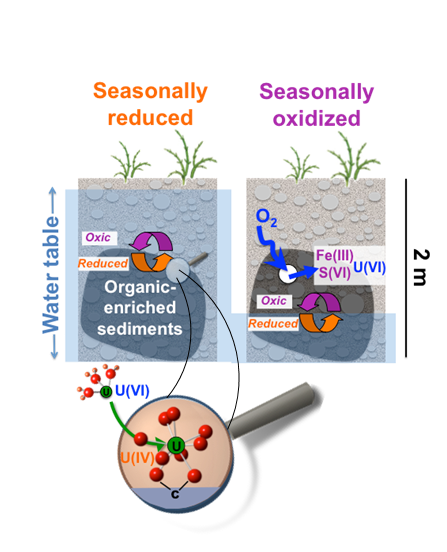 A regional model for uranium redox and mobility
A regional model for uranium redox and mobility
Noël, V.; Boye, K.; Dynes, J.; Lezama-Pacheco, J. S.; Bone S.; Janot, N.; Cardarelli, E.; Williams, K. H.; Bargar, J. R. Redox constraints over U(IV) mobility in the floodplains of Upper Colorado River basin. Environmental Science & Technology. 2017b, 51, 10954-10964.
https://pubs.acs.org/doi/full/10.1021/acs.est.7b02203
The Science
Two new studies shed light on an important and previously underappreciated biogeochemical redox ‘engine’ believed to mediate groundwater quality in floodplains within the upper Colorado River Basin (CRB). Sediments enriched in organic carbon were found to be common within saturated zones and capillary fringes, to be highly redox active, and to strongly accumulate sulfide and uranium. Read more >
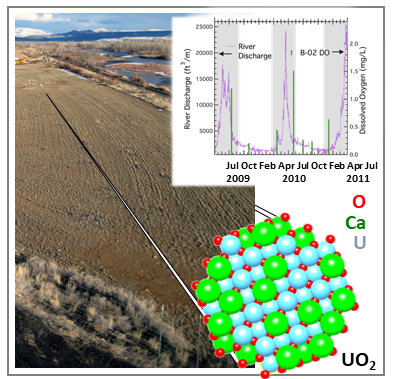 Long-Term in Situ Oxidation of Biogenic Uraninite in an Alluvial Aquifer: Impact of Dissolved Oxygen and Calcium
Long-Term in Situ Oxidation of Biogenic Uraninite in an Alluvial Aquifer: Impact of Dissolved Oxygen and Calcium
J.S. Lezama-Pacheco, J. Cerrato, H. Veeramani, D.S. Alessi, E.I. Suvorova, R. Bernier-Latmani, D.E. Giammar, P.E. Long, K.H. Williams, and J.R. Bargar, Long-Term in Situ Oxidation of Biogenic Uraninite in an Alluvial Aquifer: Impact of Dissolved Oxygen and Calcium. Environ. Sci. Technol. 2015, 49, 12, 7340–7347
https://doi.org/10.1021/acs.est.5b00949
The science.
Oxidative dissolution of U(IV) to U(VI) controls the release of uranium from reduced sediments to groundwater. In this study, uraninite (UO2) was used as a proxy for all forms of subsurface U(IV) in order to study oxidative dissolution of U(IV) under bona fide aquifer conditions. Read more >

