__________________________________________________________________________
SSRL Headlines
Vol. 9, No. 11 May, 2009
__________________________________________________________________________
Contents of this Issue:
__________________________________________________________________________
1. Science Highlight —
Bromine-rich Tips of Calcified Crab Claws as Model for Biomaterials
(contact: R. Scott, rscott@uga.edu)
|  |
The tips of crab pincers are made of a very hard material, allowing the crab to
fight and forage for food without wearing down their tools. Made mostly of
proteins, the tip is known to be bromine-rich instead of calcium-rich, but the
biochemical structure and mechanical properties were mostly unknown.
Understanding this hard material made by crabs may lead to development of
materials made by humans for use in a variety of purposes that require a small,
fracture-resistant tool.
A team led by Robert Schofield of the University of Oregon and Robert Scott of
the University of Georgia in Athens studies the material of crab claw tips.
They found that the uncalcified, bromine-rich material is harder and stiffer
than normal arthropod cuticle that lacks heavy elements and much more resistant
to fracture than the calcified material in other parts of the crab.
Theorizing that the resistance to fracture comes from the arrangement of
bromine atoms in the protein structure, the team collaborated with SSRL
scientist Matthew Latimer to analyze the distribution and environment of
bromine atoms in the crab claw tip. Using x-ray microscopy and spectroscopy at
SSRL Beam Line 9-3, they found the bromine is evenly dispersed and likely bonds
to phenyl rings, suggesting that a bromotyrosine moiety may be central to the
special properties of this material. They hypothesize that either the bromine
stiffens the material by creating more cross-links between the protein residues
or the presence of the heavy atoms dampens energies created in impacts through
their high electron densities. The results are published in the June issue of
the Journal of Structural Biology.
To learn more about this research see the full scientific highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/crab_claws.html
2. Science Highlight —
Visualizing Brain Metals in Health and Disease
(contact: H. Nichol, h.nichol@usask.ca)
| 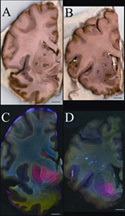 |
Metals such as iron, copper, and zinc are critical for brain function, where
they serve various roles, such as enzyme cofactors or neurotransmitters.
Because the metal atoms can be reactive and cause cell damage, their locations
and concentrations are tightly controlled. This control can be lost in some
neurodegenerative diseases. Diseases like Alzheimer's dementia and Parkinson's
disease are either caused by or lead to increased metal in areas of the brain,
while others, like Menkes disease, are characterized by decreased
concentrations of metals. Spinocerebellar ataxia (SCA) refers to a group of
degenerative disorders that cause uncoordinated movement. Some cases of SCA
are linked to abnormal metal concentrations in the brain, but the specific
patterns associated with various forms of the disorder are unknown.
A research group led by Helen Nichol from the University of Saskatchewan used
SSRL Beam Line 10-2 to compare the concentrations of iron, copper, and zinc
metals in various regions of normal and SCA-affected brain tissues. The
recently upgraded beam line allows measurement of the concentration of multiple
metals in the same whole tissue sample using a rapid scanning x-ray fluorescence
imaging technique.
In the March 24 issue of the journal Cerebellum, the researchers report
areas of both higher or lower metal concentrations in the SCA brain compared to
the control. Part of the basal ganglia had more iron. Part of the brainstem
lacked copper. Part of the cerebellum lacked iron. Some of these results were
consistent with findings from other experiments, while other observations
highlight areas warranting further study. The rapid scanning XRF technique
this team pioneered will be useful in future research of SCAs and other
neurodegenerative diseases.
To learn more about this research see the full scientific highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/brain_imaging.html
3. Science Highlight —
Potential Implications for Cataract Formation - Redox
Changes at the Sulfur Atom of Methionine
(contact: P. Kennepohl, pierre@chem.ubc.ca)
 |
In a similar way to your old pick-up truck rusting in the driveway, your body
experiences a continuous battle against the elements. A constant barrage of
oxidative stress attacks your cells and their constituent parts, including
proteins. Like rust-proof paint on your vehicle, you have defense mechanisms
that seek to prevent damage before it starts. But also like your trusty truck,
once a weakness in the armor presents itself, it can spread rapidly - and often
unnoticed - until you suddenly discover significant damage. Numerous diseases,
as well as aging itself, are linked to uncontrolled oxidative processes that
lead to irreversible damage and ultimately death. Understanding these
oxidative processes may lead toward stopping and possibly even reversing
damage.
Over the last decade, extensive studies of oxidative damage to proteins have
shown that certain amino acids are more susceptible to oxidation than others.
Methionine (MetS) is easily oxidized into its sulfoxide form (MetSO). This
damaged form is readily reduced back to its natural form by enzymes, but
sometimes it can build up and become further oxidized to sulfone
(MetSO2), for which there is no easy way back to its original MetS
form. MetS oxidation plays an important role in age-related cataracts. The
most abundant protein in the eye lens, a-crystallin,
aggregates and causes a marked decrease in the transparency of the lens -
eventually leading to blindness. These protein aggregates show signs of
oxidative damage, especially through oxidized forms of MetS. Long-term UV
light exposure has been shown to increase oxidation of
a-crystallin at methionine residues.
A research team led by Pierre Kennepohl of the University of British Columbia
explored the basic photochemical processes of MetS that may lead to damage in
lens proteins such as a-crystallin. This study,
which relied heavily on x-ray absorption spectroscopy studies of the sulfur
atoms (at the sulfur K-edge), performed at SSRL's BL6-2, showed that
light-driven processes can lead to MetS oxidation but also to beneficial
reductive processes that transform MetSO back to MetS. Which of these reaction
directions is stronger is directly linked to the presence of dioxygen
(O2). The crystalline lens is usually free of O2,
ensuring that oxidative damage can be reversed when our eyes our exposed to
light. However, lens levels of O2 are believed to increase with
age, thus acquiring the conditions that allow age-related cataracts to form.
These studies suggest that controlling the permeability of the crystalline lens
towards dioxygen could prevent age-related cataracts. So the surface of the
eye lens may, in fact, be quite similar to the rust-proof paint on your pickup.
It may work well at first, but over time it can fail, allowing rust to set in.
This work was published in the February 18 issue of the Journal of the
American Chemical Society.
To learn more about this research see the full scientific highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/mets_oxidation.html
4.
JCSG Update
(contacts: A. Deacon, adeacon@slac.stanford.edu; H-J. Chiu, chiu@slac.stanford.edu)
The Joint Center for Structural Genomics (JCSG) was established in 1999, as a consortium that
initially involved Stanford Synchrotron Radiation Lightsource (SSRL), The
Scripps Research Institute (TSRI, the lead institution), the
University of California at San Diego (UCSD), and the Genomics Institute of the Novartis
Research Foundation (GNF). The JCSG is one
of the four large-scale structural genomics production centers that are
currently funded by the NIH Institute of General Medical Sciences (NIGMS) Protein Structure Initiative (PSI). The major goal of JCSG
has been to develop and integrate innovative technologies into a highly
efficient production pipeline for protein structure determination and during
the current phase to continue development while using the pipeline for high
throughput study of the structure and function of proteins of biological
significance. The protein targets that are being actively investigated at the
JCSG include members of large protein families that have no or very little
structural coverage, proteins from metagenomes including the Global Ocean
Sampling survey and the human gut microbiome, and all proteins from the T.
maritima genome.
The Structure Determination Core (SDC) of JCSG is based at SSRL, and is
responsible for crystal screening, structure determination, structure
refinement, PDB deposition and some functional studies within the JCSG
pipeline. At SDC, all protein crystals are initially screened and their
diffraction properties are evaluated using the automation features available on
SSRL macromolecular crystallography beam lines. Thus, the best crystal of each
protein target is selected for MAD data collection. The process is very
efficient; for example, during the period of May 2008 to April 2009, the JCSG
collected 285 MAD/SAD data sets of which 86% had structures determined (i.e.
245 solved structures) that translated to 216 unique protein structures. In
addition, many high resolution native data sets and anomalous data sets used to
identify and locate the unknown bound metals in JCSG structures were also
collected over this same period. As of 05/20/2009, JCSG had screened over
100,000 crystals using the SSRL structural biology beam lines equipped with the
Stanford AutoMounter (SAM), had solved 945 structures and had deposited 838
structures in the PDB. On April 23-24 2009, over 80 JCSG consortium members,
close collaborators, members from the Scientific Advisory Board and
representatives of the NIH NIGMS PSI program met together for the JCSG's 8th
annual meeting in La Jolla, California. The presentations in the meeting
included the progress reports for the last year, and future challenges and
planning sessions for the next phase of the PSI (PSI: High Throughput
Structural Biology).
5.
A 35th Anniversary for X-ray Science at SLAC
May 4, 2009 SLAC Today Article by Shawne Workman
 |
| April 1974
drawing of
plans for the first Stanford Synchrotron Radiation Project beam lines.
|
Thirty-five years ago the Stanford Synchrotron Radiation Project - the
precursor to today's Stanford Synchrotron Radiation Lightsource - began
operations. The SLAC archives note that SSRP was the world's first synchrotron
radiation hard x-ray lightsource based on an electron storage ring. SSRP
science launched with five experimental stations sharing SLAC's first x-ray
beam line.
For more about the SSRP, see the SLAC Archives and History Office SSRP timeline
and photo gallery.
http://www.slac.stanford.edu/history/ssrp.shtml
http://www.slac.stanford.edu/history/ssrpphotos.shtml
6.
Joint SSRL/LCLS Annual Users' Conference and Workshops, October 19-21, 2009
(contact: C. Knotts, knotts@slac.stanford.edu)
Make plans to attend the 2009 SSRL/LCLS Users' Conference, October 19-21, 2009.
The meeting will have plenary talks, a joint poster session, workshops, and
vendor exhibits. This year Sebastien Boutet (sboutet@slac.stanford.edu),
Katherine Kantardjieff (kkantardjieff@exchange.fullerton.edu), Richard W. Lee
(rwlee@berkeley.edu) and Donghui Lu (dlu@slac.stanford.edu) are co-chairing the
conference. Stay tuned for more information which will be posted on the
meeting web site as it becomes available. http://www-conf.slac.stanford.edu/ssrl-lcls/2009/default.asp
7.
SLAC to Join New Energy Frontier Research Efforts
May 18, 2009 SLAC Today Article by Michael Wall
The Department of Energy has funded 46 new projects that will investigate ways
to make the U.S. energy economy greener and more secure. SLAC will contribute
substantially to at least three of these Energy Frontier Research Centers.
Each of the 46 EFRCs, which the White House announced April 27, will receive
between two and five million dollars per year for five years-a total DOE
commitment of $777 million. One project, run out of the National Renewable
Energy Laboratory in Golden, Colorado, will use the Stanford Synchrotron
Radiation Lightsource at SLAC to help identify more efficient materials for
solar energy conversion. Another, based at Oak Ridge National Laboratory, will
try to find new ways to develop super-strong, radiation-tolerant materials,
with possible applications in energy infrastructure. The Oak Ridge center will
employ SLAC's Linac Coherent Light Source in this effort. Read more at:
http://today.slac.stanford.edu/feature/2009/efrc.asp
8.
House Science Committee Staff Tour SLAC
May 29, 2009 SLAC Today Article by Shawne Workman
 |
|
SLAC Members of the House Science Committee staff hear about SLAC science from
SSRL Senior Staff Scientist Uwe Bergman. (Photo by Nicholas Bock.)
|
Yesterday afternoon, four staff members from the House Science Committee
visited SLAC for a tour of science programs and facilities across the lab.
After a luncheon with the SLAC Executive Council, the group made stops and
heard presentations at the SLAC linac, Stanford Synchrotron Radiation
Lightsource, Linac Coherent Light Source and the Kavli Institute for Particle
Astrophysics and Cosmology. The visiting science committee members were:
physicist and former American Physical Society fellow Adam Rosenberg,
professional staff member for the Energy and Environment Subcommittee of the
Full House Committee on Science and Technology; Jetta Wong, who handles
Civilian Research, Development and Demonstration Programs at the Department of
Energy; Margaret Caravelli, from the Science and Technology Committee's
Republican Staff Chief Counsel; and Professional Staff Member Elizabeth Chapel.
9.
SSRL User Admin Update
(contacts:
C. Knotts,
knotts@slac.stanford.edu; L. Dunn, lisa@slac.stanford.edu)
The next deadline for beam time proposals on our macromolecular crystallography
beam lines is July 1, 2009. Beam time eligibility for proposals submitted for
this deadline begins fall 2009. For more information see:
http://www-ssrl.slac.stanford.edu/userresources/px_proposal_guide.html
Reminder: SSRL's current experimental run will end the morning of Monday,
August 10. We expect to resume user operations in late October 2009
http://www-ssrl.slac.stanford.edu/userresources/documents/08-09_run.pdf
As always, we appreciate feedback on your data collection experience at SSRL.
We have tried to make it easier for users to review scheduled experiments and
to provide feedback by adding End of Run Summary forms to our user portal, see:
https://www-ssrl.slac.stanford.edu/URAWI/
 |
| PULSE
School |
10.
Photon Science-Related Workshops, Conferences and Schools
11.
New Luxury Hotel and Restaurant Adjacent to SLAC Site
The Rosewood Sand Hill, a luxury hotel and spa owned by Stanford and situated
on 16 acres of university land just west of SLAC, celebrated its grand opening
April 2. Rosewood Sand Hill has 123 rooms, suites and villas.
http://www.rosewoodsandhill.com/
The complex also includes a new restaurant, Madera, which features an open
artisan wood-burning kitchen, extensive wine list, comfortably elegant
ambiance, picturesque views, indoor and outdoor dining, and two private dining
rooms with terraces and cozy fireplaces. This Menlo Park restaurant is open
daily for breakfast, lunch and dinner and for Sunday brunch as well.
http://www.maderasandhill.com/
__________________________________________________________________________
SSRL Headlines is published electronically monthly to inform SSRL users,
sponsors and other interested people about happenings at SSRL. SSRL is a
national synchrotron user facility operated by Stanford University for the
U.S. Department of Energy Office of Basic Energy
Sciences. Additional support for
the structural biology program is provided by
the DOE
Office of Biological and Environmental Research, the NIH
National Center for Research Resources and the NIH Institute for General Medical
Sciences. Additional information about
SSRL and its operation and schedules is available from the SSRL WWW
site.
__________________________________________________________________________
To leave the SSRL-HEADLINES distribution, send email as shown below:
To: LISTSERV@SSRL.SLAC.STANFORD.EDU
Subject: (blank, or anything you like)
The message body should read
SIGNOFF SSRL-HEADLINES
That's all it takes. (If we have an old email address for you that is
forwarded to your current address, the system may not recognize who
should be unsubscribed. In that case please write to
ssrl-headlines-request@ssrl.slac.stanford.edu and we'll try to figure out
who you are so that you can be unsubscribed.)
If a colleague would like to subscribe to the list, he or she should send
To: LISTSERV@SSRL.SLAC.STANFORD.EDU and use the message body
SUBSCRIBE SSRL-HEADLINES
|











