

 |
|
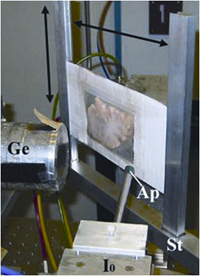
| Fig. 1. Rapid-scanning x-ray fluorescence mapping ex perimental setup. Synchrotron x-rays at 11 keV passed through a 50 Ám aperture (Ap). The beam intensity was monitored with a N2-filled ion chamber (I0). The brain slice was mounted vertically on a motorized stage (St) at 45° to the incident x-ray beam and raster scanned in the beam. A 13-element Ge detector (Ge) was positioned at a 90° angle to the beam. |
In an article published in the March 24 issue of Cerebellum, Popescu and
co-workers have provided new insight into which brain metals are dysregulated
in spinocerebellar ataxia (SCA). SCAs are a group of degenerative disorders
that affect the cerebellum, the spinal cord and the connections between them
causing a type of uncoordinated movement called ataxia. Friedreich's ataxia,
ataxia with isolated vitamin E deficiency, X-linked sideroblastic anemia with
ataxia, ataxia telangiectasia and infantile-onset spinocerebellar ataxia are
examples of SCAs that have been linked to metal abnormalities. Each of these
conditions has a different cause and thus, each may have a different pattern of
metal dysregulation.
Rapid scanning X-ray fluorescence imaging [5], conducted on
SSRL Beam Line 10-2, was used to compare the normal distribution of iron,
copper and zinc to their distribution in a case of SCA. Formalin-fixed slices
of brain and spinal cord, approximately 1 cm thick, were purchased from the
Brain and Tissue Bank
for Developmental Disorders at the University of Maryland, a facility funded by
In the SCA sample, the forebrain did not show degenerative changes but the
distribution of metals was unusual (Fig. 2). The basal ganglia are huge brain
nuclear masses that integrate and regulate the excitatory and inhibitory
circuitry adjusting the movement, amongst other functions. The globus pallidus
par externa (GPe) is part of the basal ganglia involved in movement inhibition.
The GPe of the SCA brain had about 80% more iron than the GPe of the normal
brain. The importance of GPe iron in the development or potential treatment of
SCA remains to be determined since no obvious cellular degeneration was seen in
the GPe.
The cerebellum (Fig. 4) is quite rich in metals and contains one of the most
metal-rich structures in the brain, the dentate nucleus [7].
The striking branching pattern of the cerebellar white matter called the
arbor vitae (tree of life) is clearly distinguished on the basis of its
metal content. In SCA the iron content of the dentate nucleus is much lower
than that seen in the control brain, and the arbor vitae is not as
prominent.
The high metal content of the dentate nucleus, white matter of the cerebellum
and the medullary 'olive' makes these brain regions particularly susceptible to
metal-catalyzed oxidative damage, neurotoxicity and neurodegeneration should
metal metabolism become dysregulated. This study shows that neurodegeneration
Beam line 10-2 has recently been upgraded with a new set of motorized stages
which allow rapid collection of x-ray fluorescence data on large samples (up to
300 mm x 600 mm) and new fluorescence x-ray digital signal processors. This
new data collection system also allows rapid collection of data points, the
collection of the full x-ray fluorescence spectrum at every pixel, and the
ability to return to points of interest easily to further collect x-ray
absorption spectra. These and other upgrades in the future will be utilized
for further studies on SCAs and other neurodegenerative diseases.
Primary Citation
Popescu, B., F., Gh, Robinson, C.A., Chapman, L.D., Nichol, H. (2009)
Synchrotron X-ray Fluorescence Reveals Abnormal Metal Distributions in the
Brain and Spinal Cord in Spinocerebellar Ataxia: A Case Report.
Cerebellum, Mar
24 (Epub ahead of print) DOI 10.1007/s12311-009-0102-z.
the National Institute of Child Health and Development. The brain and spinal
cord slices were sealed without further treatment under metal-free plastic or
Mylar and raster-scanned in the beam (Fig. 1). Areas of interest were
subsequently excised for histological and immunohistochemical analysis.
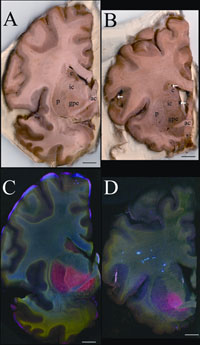
Fig. 2.
Metals are increased in the globus pallidus pars externa; A. Gross section of
the control forebrain; B. Gross section of the SCA forebrain; C. Overlay of Fe
(red), Cu (green) and Zn (blue) distribution of the control forebrain; D.
Overlay of Fe (red), Cu (green) and Zn (blue) distribution of the SCA
forebrain; c caudate; p putamen; gpe globus pallidus pars externa; ic internal
capsule; ac anterior commissure; arrows blood vessels; arrowheads metal rich
white matter regions; scale bar=5 mm.
The brainstem connects the forebrain to the spinal cord. In the part of the
brainstem called the medulla is an important movement coordination center named
after its shape "the olive". Popescu et al. found that the nerve cell
processes that pass from this region to the cerebellum are exceptionally rich
in copper and this had not been previously described (Fig. 3). In contrast to
the normal medulla, the 'olive' of the SCA medulla was nearly devoid of copper.
It is not known why these fibers are normally rich in copper but they lack
copper in SCA. However, it is interesting to note that intoxication with
clioquinol, an antibiotic and potent copper chelator, causes characteristic
degeneration in this brain region [6].
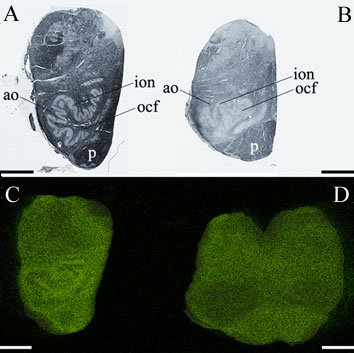
Fig. 3.
Cu is abundant in the control olive and decreased in the degenerated olive of
the SCA patient; A Normal histological appearance of the control medulla (Luxol
fast blue/cresyl violet staining); B Degenerated olive in the SCA medulla
(Luxol fast blue/cresyl violet staining); C. Cu map of the control medulla; D.
Cu map of the SCA medulla; ion inferior olivary nucleus; ocf olivocerebellar
fibers; ao amiculum olivae; p pyramids; scale bar=5 mm.
can be linked to either metal accumulation or reduction depending upon the
brain region and the stage of neurodegeneration. The ability to map multiple
metals in whole human brain gives us a new tool to examine metal pathologies in
a variety of diseases affecting the brain and spinal cord.

Fig. 4.
Iron is decreased in the degenerated dentate nucleus and metals are decreased
in the cerebellar white matter of the SCA patient; A Gross section of the
control cerebellum; B Gross section of the SCA cerebellum; C. Overlay of Fe
(red), Cu (green) and Zn (blue) distribution of the control cerebellum; D.
Overlay of Fe (red), Cu (green) and Zn (blue) distribution of the SCAl
cerebellum; dn dentate nucleus; wm white matter; gm cortical gray matter; scale
bar=5 mm.
References
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.