

 |
|
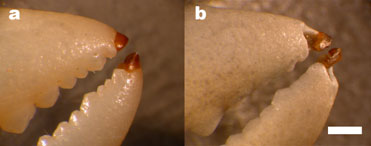
| Figure 1. The heavy element biomaterial is the darker material at the tip of the shore crab claws. Figure "b" shows the same claw as "a" but after bead blasting. The claw tips are less eroded by the bead blasting than surrounding calcified material, suggesting a greater resistance to chipping from impact. Scale bar: 2mm | |
Our results suggest that the greatest advantage of bromine-rich cuticle over calcified cuticle is resistance to fracture (the energy of fracture is about an order of magnitude greater than for calcified cuticle). The greatest advantage relative to unenriched cuticle, was a factor of about 1.5 greater hardness and modulus of elasticity, making the tips harder and more stiff than acrylic glass. The claw tips gain increased fracture resistance from the orientation of the constituent laminae and from the viscoelasticity of the materials.
It may not be surprising that smaller organisms would employ fracture-resistant materials in the thin regions of their "tools". Smaller organisms may be subjected to the same forces from the environment, predators, and prey as larger organisms, but the smaller cross sections of their "tools" make them more susceptible to fracture.
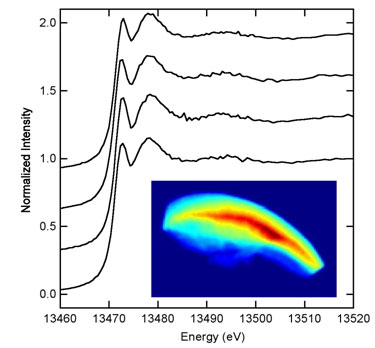 | |
| Figure 2.The inset is an image of the bromine Ka fluorescence emission intensity [in arbitrary units ranging from zero (dark blue) to highest (dark red)] recorded on a crab claw tip specimen by scanning fluorescence x-ray microscopy with excitation at 14 keV. The x-ray beam size was approx. 10 x 10 mm and the raster step size was 10 mm. The horizontal dimension of this image is 2.0 mm. The upper convex edge of the bromine emission coincides with the external surface of the crab claw tip while the bottom represents the dissection region. Four spots across the bromine content of this specimen were selected to collect bromine XAS edge spectra and these spectra (single first sweeps) are displayed above the inset image. The shape and structure of these edges are sensitive to the local bromine environment and all appear identical to one another and very similar to the edge observed in the bulk bromine XAS experiment, suggesting a homogeneous bromine environment. | |
The mechanism by which the bromine (and other heavy elements in other organisms) modifies the mechanical properties is not understood. One possibility is that bromine makes the material harder and stiffer by increasing the cross-links between proteins. Another, more speculative, possibility is that the high mass density of the heavy elements improves resistance to fracture. The attachment of many heavy bromine atoms to many phenyl rings along the protein would reduce the resonant frequencies of certain low frequency large-scale molecular motions (such as standing torsional waves over the length of the molecule). If these resonances were lowered to overlap more with the range of frequencies associated with impacts, then bromination might improve damping of impact energy.
A good example of the bromine biomaterial can be investigated the next time you
eat a Dungeness crab. Notice that the sharp tip of the leg is a cap of
translucent material that is very different from the rest of the crab. It is
very difficult to break the tip, even though it is very thin, stiff and hard.
Humans are just starting to try to engineer tiny machines and tools, and we
have a lot still to learn from organisms that have coped with being small for
millions of years.
Primary Citation
R. M. S. Schofield, J. C. Niedbala, M. H. Nesson, Y. Tao, J. E. Shokes, R. A.
Scott and M. J. Latimer, "Br-rich Tips of Calcified Crab Claws are Less Hard
but More Fracture Resistant: A Comparison of Biomineralized and Heavy-element
Biomaterials", J. Struct. Biol. 166, 272 (2009)
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.