__________________________________________________________________________
SSRL Headlines Vol. 7, No. 5 November, 2006
__________________________________________________________________________
Contents of this Issue:
__________________________________________________________________________
1. Science Highlight —
Untangling Brain Disease
(contacts: X. Wang, wxq@stanford.edu; K.C. Garcia, kcgarcia@stanford.edu)
Researchers have for the first time obtained a high-resolution structure of a
three-molecule receptor-ligand complex that could help shed light on
neurodegenerative diseases such as Parkinson's. The complex includes two
receptor molecules, called GFRa3, bound with its
ligand, artemin, which fit together like a lock and key. These molecules play a
key role in chemical signal transmission and in the development and health of
neurons.
This research was the result of macromolecular crystallography data measured at
SSRL BL11-1 and at the Advanced Light Source in Berkeley. Researchers collected
x-ray diffraction data from two types of crystals- the
artemin-GFRa3 bound
complex, and artemin by itself - overcoming the inherent difficulty of growing
receptor molecules such as GFRa3 to into crystals.
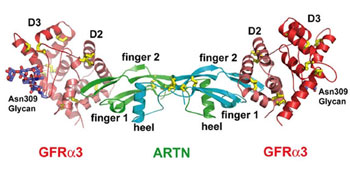
|
|
Overall structure the ARTN-GFRa3 complex in ribbon
representation. |
|
Glia are important nervous system cells that help regulate the internal
environment in the brain. Artemin is one of four compounds belonging to a
family of ligands known as glial cell line-derived neurotrophic factor (GDNF),
which are responsible for maintaining the health of dopaminergic and
motorneurons. Other ligands in this family include GDNF itself, neurturin, and
persephin. GDNF and its receptor GFRa1 have
been linked to neurodegenerative
diseases such as Parkinson's disease, but have so far defied crystallization.
The artemin-GFRa3 complex is structurally similar to
GDNF-GFRa1, and therefore determining the structure
of this related complex could lead to the development of new therapies for
neurodegenerative diseases.
To learn more about this research see the full technical highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/artn.html
2. Science Highlight —
Science Highlight - Learning How Nature Splits Water
(contacts:
J. Yano, jyano@lbl.gov; V.K. Yachandra, vkyachandra@lbl.gov)
|  |
| This image portrays the
water-splitting catalytic cycle with the Mn4Ca structure in the
middle.
|
Billions of years ago, primitive bacteria developed a way to harness sunlight
to split water molecules into protons, electrons and oxygen-the cornerstone of
photosynthesis. Now, a team of scientists has taken a major step toward
understanding this process by deriving the precise structure of the catalytic
metal-cluster center containing four manganese atoms and one calcium atom
(Mn4Ca) that drives this water-splitting reaction. This catalytic
center resides in a large protein complex, called photosystem II, found in
plants, green algae, and cyanobacteria. The international team was led by
scientists from LBNL, and includes scientists from Germany's Technical and Free
Universities in Berlin, the Max Planck Institute in Mülheim, and from SSRL.
Until now, the precise structure of the Mn4Ca cluster has eluded all
attempts of determination by x-ray crystallography and spectroscopic
techniques, in part because the metal catalyst center is highly susceptible to
radiation damage. The team used a novel combination of polarized single crystal
x-ray absorption spectroscopy (XAS) and x-ray diffraction measurements at
SSRL's BL9-3 to control the radiation dose and thereby obtain XAS data to high
resolution. This enabled the team to constrain the possible metal cluster site
structure to three similar ones at a resolution much higher (~0.15 Ċ) than
previously possible.
The work, detailed in the Nov. 3, 2006 issue of the journal Science,
could help
researchers synthesize molecules that mimic this catalyst, which is a central
focus in the push to develop clean energy technologies that rely on sunlight to
split water and form hydrogen to feed fuel cells or other non-polluting power
sources.
To learn more about this research see the full technical highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/psII-06.html
3. Science Highlight —
Science Highlight - Femtosecond Diffractive Imaging with a Soft-X-ray FEL
(contacts:
H. Chapman, chapman99@llnl.gov; J. Hajdu, janos@slac.stanford.edu)
 | |
| The diffraction pattern
recorded with the second pulse, showing diffraction from the hole in the sample
created by the first pulse.
| |
Scientists have for the first time used an extremely short and intense coherent
soft x-ray laser pulse to successfully obtain a high-resolution image of a
nano-scale object before the sample was destroyed by the energy impact of the
pulse. The experiment, conducted at Deutsches Elektronen-Synchrotron (DESY) in
Hamburg by a collaboration that included researchers from the Photon Science
Directorate at SLAC, also set a speed record of 25 femtoseconds for the
duration of the x-ray pulse used to acquire the image. The results are
published in the November 12 online edition and the December printed edition of
Nature Physics.
Using the soft x-ray free-electron laser FLASH at DESY at a 32 nm wavelength,
the international collaboration led by Janos Hajdu (SLAC and Uppsala
University) and Henry Chapman (LLNL) exposed a sample that contained
nanometer-sized objects and recorded the x-ray diffraction pattern using a
novel fast detector. A special computer algorithm was then used to recreate an
image of the object based on the recorded diffraction pattern.
The technique used to capture the image is called "flash diffraction imaging,"
and this experiment proves the principle behind atomic-scale imaging that will
be applied when even more powerful x-ray free-electron lasers are available,
such as the LCLS, now under construction at SLAC; the SPring-8 SCSS facility in
Japan; and the European XFEL in Hamburg. According to researchers, these
revolutionary FELs will give scientists unprecedented insight into structural
dynamics of a variety of materials.
The work was funded in part by the U.S. Department of Energy Office of Science,
by a Laboratory Directed Research and Development strategic initiative proposal
for "biological imaging with fourth-generation light sources" at LLNL, and by
Swedish Research Councils.
To learn more about this research see the full technical
highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/flash.html
4. First Light on BL12 and Other Operations
Updates
(contact:
C. Knotts, knotts@slac.stanford.edu)
 |
|
Fluorescent screen showing first light in BL12-2 hutch. |
First Light on 12-2: Thanks to the diligent efforts of staff from just
about
every group at SSRL and many at SLAC, BL12-2 an in-vacuum undulator beam line
for macromolecular crystallography, has started beam commissioning. At 8:30
a.m. on Tuesday, November 28, the BL12 front end opened for the first time with
25 mA and K=1.73. By 10:05 a.m. unfocused monochromatic beam was threaded
through to the BL12-2 experimental hutch. Many thanks to all who contributed
to this effort over the past few years.
Beam Line Update: A new
sagittal focusing monochromator is being installed and commissioned on BL7-2.
We anticipate that this will be finished by January, so that users operations
can resume on this beam line. BL4 is scheduled for a complete upgrade for
500-mA SPEAR3 operations during the second half of calendar year 2007. In the
interim, BL4-1 and BL4-3 are closed, but BL4-2 continues to be available for
structural molecular biology small angle x-ray scattering and diffraction.
500 mA Update: Since the SPEAR3 upgrade in 2003-04, SSRL has
implemented an
aggressive schedule of beam line upgrade/development projects to make all beam
lines compatible with 500-mA operations, which is the SPEAR3 design current. By
the end of 2006, the optics, beam transport components, and shielding of 14
insertion device branch lines and 6 bend magnet branch lines will have been
upgraded for 500-mA SPEAR3 power, while the remaining 3 insertion device branch
lines are scheduled for completion by the end of 2007. Additionally, a new beam
containment monitoring system required for 500-mA beam line operations will be
phased in during the FY2007 run. We anticipate that there will be opportunities
in the second and third scheduling period in 2007 for staff tests (and limited
user experiments) utilizing higher current. If you are interested in being part
of these commissioning experiments, please let us know by including a comment
on your beam time request.
X-ray/VUV Beam time requests for the second scheduling period in 2007 (Feb-May)
are due before Friday, December 1:
http://www-ssrl.slac.stanford.edu/users/user_admin/xray_btrf.html
http://www-ssrl.slac.stanford.edu/users/user_admin/vuv_btrf.html
The beam line schedule and the 2006-2007 SPEAR3 operating schedule which runs
through August 6, 2007 are available on the SSRL website.
"http://www-ssrl.slac.stanford.edu/schedules/06-07_run.pdf
The SPEAR3 schedule includes information on scheduled maintenance, accelerator
physics studies, and holiday closures - note that SSRL and user operations on
SPEAR3 will be closed for the winter holidays from December 23, 2006-January 2,
2007.
5. X-ray Diffraction and the Fight against Heart
Disease
(contact:
A. Mehta, mehta@slac.stanford.edu)
X-ray diffraction is a very powerful technique for measuring microstrains not
only because it is one of the few techniques that can distinguish among the
four different modes of microstrain, but also because it is a non-contact probe
with large penetration depth. This allows it to be easily incorporated into an
in-situ measurement. Over the last two years we, in collaboration with
Prof.
Rob Ritchie's group at Lawrence Berkeley National Laboratory and Dr. Alan
Pelton's research group at Nitinol Devices & Components/Johnson & Johnson (a
stent manufacturer), have been using synchrotron based diffraction and
microdiffraction at SSRL and the Advanced Light Source to probe the various
modes of microstrain in mechanically simple and better defined and sometimes on
more complex stent-like Nitinol objects. See full story at:
http://today.slac.stanford.edu/feature/ROW-110206.asp
6. LCLS Lehman Review
(contact: J. Galayda,
galayda@slac.stanford.edu)
The LCLS Project had its semiannual Department of Energy Office of Science
review on 24-26 October. The review committee, chaired by Daniel Lehman,
included experts in the full spectrum of Project activities: accelerators,
undulators, x-ray experiments, civil construction, computer controls and
project management. Overall the progress of the Project was judged to be
excellent. The reviewers toured the new Undulator Magnet Measurement Facility
in Building 85, the completed LCLS Injector Facility with its newly installed
Thales laser, and the progress of Turner Construction Company at re-shaping the
Research Yard and PEP Ring Road area to build the LCLS. A subgroup entered the
linac tunnel to view the progress toward installing the Bunch Compressor 1
chicane.
Concerns and questions raised at the last review (plans for coordinated
installation of hardware for accelerator, x-ray experiments and civil
construction; results of undulator prototyping, performance of the gun laser
and the electron gun itself) were addressed to the satisfaction of the
reviewers.
As the Project progresses, new challenges may arise. Perhaps the most
significant challenge, that of a revised plan for providing office space for
LCLS operations, was addressed squarely by SLAC and DOE at the review.
Construction costs in the Bay Area have gone up very significantly during the
design period of the LCLS. While the "beam path" (electron beam transport,
undulator halls, and experiment halls) will be constructed as originally
designed, insufficient funds are available to the Project to construct the
Central Lab Office Complex as originally intended. With guidance from SLAC
management and consent from DOE, the Project will develop a new and more modest
plan for housing LCLS personnel in the next few months, with implementation to
begin in the 9/2007 timeframe.
At the next review in April, the LCLS Injector Linac will be well advanced in
commissioning. All undulators will be delivered and civil construction will be
well under way. The Accelerator Systems Division of the Particle/Particle
Astrophysics Directorate will have operational control of the Injector, the
first in several steps along the way toward handover of the new LCLS facilities
to SLAC.
7. Keith Hodgson Elected 2006 AAAS Fellow
Ten Stanford professors are among the 449 newly elected fellows of the American
Association for the Advancement of Science (AAAS), the world's largest
organization of scientists.
|
 |
|
K.O. Hodgson |
|
Photon Science Director Keith Hodgson will join Steven Block,
Henry Greely,
Chaitan Khosla, Joseph Lipsick, William Mobley, Martin Perl, Shauna Somerville,
Teresa Wang and Jeffrey Wine when they are presented with certificates and pins
on Feb. 17, during the AAAS annual meeting in San Francisco. The scientists
were chosen "because of their efforts toward advancing science applications
that are deemed scientifically or socially distinguished," according to a
statement by the AAAS Office of Public Programs in Washington, D.C.
Keith O. Hodgson, also the Howard H. and Jessie T. Watkins University Professor
of the Stanford Synchrotron Radiation Laboratory and Chemistry and the Deputy
Director of SLAC, was chosen for applications of synchrotron radiation
spectroscopy, diffraction and scattering to study structure and function
relations in bioinorganic chemistry and biophysics, in particular nitrogenase.
News Release: http://www.stanford.edu/dept/news/pr/2006/pr-aaasfel-112906.html
8. SLAC Security Gate 17 Open 24/7
The Security Gate 17 is open 24 hours, seven days a week, allowing easy access
for users and staff from outside of the gate (e.g., Guest House, cafeteria,
SSRL Building 137) to SPEAR3 and other SSRL buildings inside the accelerator
fence. The extended hours for this gate are due to LCLS construction which has
bisected the PEP Ring Road at two points: just past the Collider Hall from the
north, and at the intersection of Alpine Gate Road from the south. The area in
between these points is restricted to construction activities only. Gate 17
allows access the north side of the SLAC research yard and interaction region 2
(IR2); Gate 30 continues to remain open all day allowing access to the south
section of the research yard and interaction region 4 (IR4). The antenna tower
/ overlook road above the SPEAR3 complex are also closed to vehicles and
pedestrians, except for emergency vehicles needing access to both sides of the
research yard. And, for the duration of the LCLS construction, the back
entrance to SLAC (the Alpine Gate) is also closed to regular traffic (open only
for use by construction vehicles and heavy equipment).
 | |
|
Stanford Guest House
| |
9. Changes to On Site Guest House
The Guest House will be closed over the winter holidays from December 16, 2006
until January 2, 2007. When the facility reopens in January, new rates will
take effect for SSRL and SLAC guests: standard Room (with one full bed or bunk
beds) will be $75.00 per night plus tax; a larger Room (with two full beds or
one queen bed) will be $105.00 per night plus tax.
http://www.stanford.edu/dept/hds/guesthouse/
10.
Photon Science Job Opportunities
A number of positions are currently available at the LCLS, LUSI and SSRL.
Please refer to the Photon Science Job Openings page for more information about
these job opportunities.
http://www-ssrl.slac.stanford.edu/photonscience/jobs.html
__________________________________________________________________________
SSRL Headlines is published electronically monthly to inform SSRL users,
sponsors and other interested people about happenings at SSRL. SSRL is a
national synchrotron user facility operated by Stanford University for the
U.S. Department of Energy Office of Basic Energy
Sciences. Additional support for
the structural biology program is provided by
the DOE
Office of Biological and Environmental Research, the NIH
National Center for Research Resources and the NIH Institute for General Medical
Sciences. Additional information about
SSRL and its operation and schedules is available from the SSRL WWW
site.
__________________________________________________________________________
To leave the SSRL-HEADLINES distribution, send email as shown below:
To: LISTSERV@SSRL.SLAC.STANFORD.EDU
Subject: (blank, or anything you like)
The message body should read
SIGNOFF SSRL-HEADLINES
That's all it takes. (If we have an old email address for you that is
forwarded to your current address, the system may not recognize who
should be unsubscribed. In that case please write to
ssrl-headlines-request@ssrl.slac.stanford.edu and we'll try to figure out
who you are so that you can be unsubscribed.)
If a colleague would like to subscribe to the list, he or she should send
To: LISTSERV@SSRL.SLAC.STANFORD.EDU and use the message body
SUBSCRIBE SSRL-HEADLINES