

We have demonstrated flash diffractive imaging of nanostructures using pulses
from the first soft-X-ray free-electron laser in the world, FLASH. This is the
first step in the development of single-molecule diffractive imaging (Neutze
et al., Nature, 406, pp. 752-757, 2000), where atomic-resolution
images of macromolecules will be obtained, without the need for crystallization
of the molecules.
As reported in the December issue of Nature Physics, we used an intense,
ultrafast pulse from the FLASH soft-X-ray free-electron laser to record a
coherent X-ray diffraction pattern from a nanoscale object. We inverted this
pattern to form a high-resolution image of the object, without the need for any
lenses, using the Shrinkwrap algorithm (Marchesini et al, Phys Rev
B, 68, 140101(R) 2003). The flash image resolves 50 nm features,
was recorded in 25 fs, and is the fastest image ever recorded with sub-optical
resolution. The 25 fs pulse contained about 1012 photons and was
focused down onto the sample to give an intensity of 4x1013
W/cm2, at a wavelength of 32 nm. The coherent diffraction pattern
was obtained before the sample exploded at a temperature of 60,000 K. No
evidence of sample damage could be seen in the reconstructed image.
We measured the diffraction pattern with a sensitivity of single photon
detection, even though the sample radiates after explosion and the intense
pulse vaporizes anything else in its path. This was achieved with a novel
diffraction camera consisting of a graded multilayer mirror which varies in
period by a factor of two across its face. The direct beam passed through a
hole in the mirror, while the coherent scattering from the sample was reflected
by the mirror onto a CCD. The mirror efficiently filtered out radiation of
other wavelengths and radiation travelling in the wrong direction (i.e. not
emanating from the sample).
Today, the bottleneck in the atomic resolution imaging of large macromolecules
and macromolecular complexes is a fundamental need for crystals. This limits
the scope of detailed structural analysis to molecules and assemblies which can
be crystallized. Many biologically important target complexes are difficult or
impossible to crystallize. There is, therefore, a great need to develop new
structural determination methods.
It was suggested by Neutze et al. (Nature, 406, 752-757, 2000)
that ultrafast pulses from a free-electron laser could be intense enough to
give a measurable diffraction pattern from a single uncrystallized
macromolecule. The incident X-ray dose would exceed the usual tolerance of
biological materials to keep their structural integrity, by five orders of
magnitude. In fact, the interaction with the pulse would be so violent that
the molecule would completely vaporize. However, due to the inertia of the
atoms, this damage process would not start until after the pulse had carried
away the information of the undamaged molecule.
To build up a three-dimensional image requires many coherent diffraction
patterns from many, identical, macromolecules. There are many challenges yet
to be addressed to carry out single-molecule imaging, such as delivering the
samples, orienting the diffraction data (or the molecules) and combining the
data. However, this current work gives great hope to the method, as it was
demonstrated that it is possible to record a diffraction pattern, with single
photon sensitivity, even when the illuminating pulse destroys everything in its
path, and turns the sample into a radiating plasma. The image was
reconstructed at the highest resolution consistent with the illumination and
collection aperture of the detector.
The experiments were carried out by a collaborative team from Lawrence
Livermore National Laboratory, the NSF Center for Biophotonics Science and
Technology, Stanford Synchrotron Radiation Laboratory, Uppsala University, the
Deutsches Elektronen-Synchroton (DESY), and Technische Universität Berlin. The
FLASH FEL began operations at DESY in August, 2005.
Primary Citation
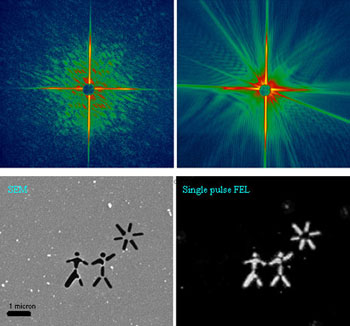
Top left: A diffraction pattern recorded with a single FLASH
FEL pulse from a test object placed in the focused beam. Top right: The
diffraction pattern recorded with the second pulse, showing diffraction from
the hole in the sample created by the first pulse. The sample was a pattern
milled from a 20-nm thick silicon nitride membrane, shown Bottom left.
Bottom right: The image reconstructed from the single-shot diffraction pattern
using our "Shrinkwrap" phase retrieval algorithm. The algorithm only used the
measured diffraction intensities and the knowledge that the diffraction pattern
was oversampled. We did not use the SEM image in the reconstruction.
H. N. Chapman, A. Barty, M. J. Bogan, S. Boutel, M. Frank, S. P. Hau-Reige, S.
Marchesini, B. W. Woods, S. Bajt, W. Henry. Benner, R. A. London, E. Plönjes,
M. Kuhlmann, R. Treusch, S. Düsterer, T. Tschentscher, J. R. Schneider, E.
Spiller, T. Möller, C. Bostedt, M. Hoener, D. A. Shapiro, K. O. Hodgson, D. van
der Spoel, F. Burmeister, M. Bergh, C. Caleman, G. Huldt, M. Seibert, F. R. N.
C. Maia, R. W. Lee, A. Szöke, N. Timneanu and J. Hajdu, "Femtosecond
Diffractive Imaging with a Soft-X-ray Free-Electron Laser", Nat. Phys.
Published online: 12 November 2006 | doi:10.1038/nphys461
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |