1. Science Highlight — Chemists Discover how Nature Makes Medicine
(contact: C.L. Drennan, cdrennan@mit.edu)
After years of wondering how organisms managed to create medically valuable natural products, like antibiotics and anti-fungal agents, chemists have discovered the surprisingly simple secret by shining x-ray light on the problem. MIT and Harvard researchers used crystallography beam lines at the Stanford Synchrotron Radiation Laboratory and the Advanced Light Source in Berkeley for their research.
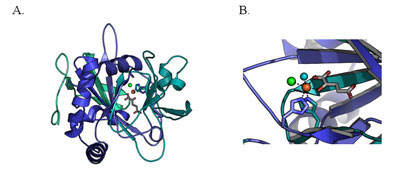
They determined the atomic structure of an iron-dependent halogenase, the enzyme SyrB2, from the plant pathogen Pseudomonas syringae. This enzyme catalyzes the chlorination of threonine during biosynthesis of the anti-fungal agent syringomycin, a natural-product antibiotic. This provides one example of how an enzyme can coax a reaction to generate medically valuable halogenated natural products. The products include antibiotics, anti-tumor agents and fungicides, and they are challenging to synthesize in a laboratory. The crystallography study revealed in this case how the specific structure at the iron-containing active site of the enzyme (the site responsible for the chemical reaction) provides information that can help in understanding how the chemical process takes place.
|
"Now that we have the enzyme's structure and figured out how it works, it makes sense. But it's not what we would have predicted," said Catherine Drennan of MIT. "Things are usually not this simple, but there's an elegant beauty in this simplicity," one that might help chemistry labs gain the enzyme's capabilities.
To learn more about this research see the full technical highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/SyrB2.html
2. Science Highlight —
Protecting against DNA Invasion
(contact:
G. Balendiran, gbalendiran@coh.org)
| ||
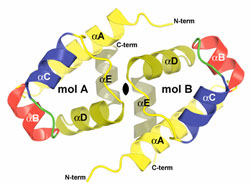
Researchers from the City of Hope cancer research and treatment center in Duarte, California, determined the crystal structure of the protein that controls this defense system in bacteria called Bacillus caldolyticus. Unless stopped, viral DNA slips into bacterial DNA, where it gets copied many times over, and then destroys its host. To protect bacterial cells, the control protein ensures the proper ratio between two enzymes, the "swords" and the "shields." The sword enzyme slashes invading viral DNA into useless pieces. The shield enzyme adds a protective layer to bacterial DNA, so the sword will not cut its master. Too few shields lead to bacterial cell death, and too many shields protect the viral DNA as well.
The crystal structure of the control protein uncovers the presence of a helix-turn-helix (HTH) motif which has the potential to bend B-DNA. The structural study also revealed amino acid residues that are most likely involved in the DNA interaction. The proposed model suggests that the protein adjusts the levels of the defense enzymes (swords and shields) by sliding along the bacterial DNA, bending and changing its shape to turn on or off the respective genes.
To learn more about this research see the full technical highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/cbcli.html
3.
SPEAR3 Fast Orbit Feedback Milestone Achieved in June
(contact:
R. Hettel, hettel@slac.stanford.edu)
On June 14, the SSRL Accelerator Systems Department commissioned an improved system designed to stabilize electron beam orbit in a frequency bandwidth approaching 100 Hz. The Fast Orbit Feedback System replaces the slower version, which corrects the orbit every few seconds, and that has been in use since SPEAR3 became operational. The new system acquires electron orbit and photon beam position data and updates the orbit correction magnets at a 4 kHz rate using a high speed distributed processing network. The system is effective in suppressing beam motion up to a few 10s of Hz caused by magnet support girder vibration, traffic near the SPEAR3 complex, and, most notably, disturbances arising from user-controlled insertion device changes. Work is continuing to improve and optimize fast orbit feedback performance.
Please take a few moments to consider nominating your colleagues for one or
more of the following awards which will be presented at the 33rd Annual SSRL
Users' Meeting, October 12-13, 2006:
Earlier this year, the SSRL Users' Organization Executive Committee (SSRLUOEC)
established a Scientific Development Award to honor Melvin P. Klein
(1921-2000), a pioneer at the forefront of accomplishments in NMR, EPR, and
x-ray absorption spectroscopy who was dedicated to the pursuit of the structure
of the Mn complex characterized by the interplay of these methods. The Melvin
P. Klein Scientific Development Award will be given to an undergraduate or
graduate student to disseminate scientific results based on work performed at
SSRL. The award (up to $1,000) will reimburse a student to present their work
at a scientific conference during the following year. The recipient will be
selected by a user subcommittee based on a nomination package which should
include a letter of recommendation from the advisor; an abstract written by the
candidate describing the experiment and scientific results (not to exceed 300
words); and information on when and where the work is to be presented.
Nominations must be received by August 1. Additional instructions and
information on making a donation toward this award are available at:
http://www-ssrl.slac.stanford.edu/kleinaward.html
Submit nominations for the William E. Spicer Young Investigator Award by August
1. The Spicer Award was established in 2004 to honor Bill Spicer (1929-2004),
one of the original founders of the Stanford Synchrotron Radiation Project.
This award recognizes important technical or scientific accomplishments that
benefited from, or are beneficial to, the SSRL. The award is open to senior
graduate students and PhDs within seven years of entry into their professional
scientific field. The award consists of a certificate and $1,000 as well as
waived registration and travel support to make a presentation at SSRL33 on
October 12-13. Nominations in the form of a letter or email summarizing the
technical or scientific contributions of the candidate must be sent to Cathy
Knotts (knotts@slac.stanford.edu) before August 1. Nominations should include
the candidate's CV and publications; supporting letters are also encouraged.
Submit nominations for 2006 Farrel W. Lytle Award by August 15. The Lytle Award
was established by the SSRLUOEC to promote important technical or scientific
accomplishments in synchrotron radiation-based science and to foster
collaboration and efficient use of beam time among users and staff at SSRL. The
award consists of a certificate and $1,000. All SSRL users and staff are
eligible for this award. The recipient will be selected by the SSRLUOEC, and
the award will be presented at the SSRL33 awards dinner on Thursday, October
12, 2006. Nominations summarizing the individual's contributions and why they
should be recognized through this award must be sent before the August 15
deadline to Cathy Knotts (knotts@slac.stanford.edu):
http://www-ssrl.slac.stanford.edu/lytleaward.html
Several months ago, SLAC began distributing an electronic daily newsletter,
SLAC Today. With additional communications staff, we are able to feature more
articles about SSRL activities written for the general public in SLAC Today as
well as in this monthly newsletter. See the following for some of the more
recent articles. To subscribe to SLAC Today, visit
July 1, 2006 is the next deadline for submitting macromolecular crystallography
proposals. Proposals submitted in July will be eligible for beam time
beginning in November 2006. For more information see Proposal Submittal and
Scheduling Procedures for Macromolecular Beam Lines at SSRL.
http://www-ssrl.slac.stanford.edu/users/user_admin/guide.html
A number of positions are currently available at the LCLS, LUSI and SSRL.
Please refer to the Photon Science Job Openings page for more information about
these job opportunities.
__________________________________________________________________________
SSRL Headlines is published electronically monthly to inform SSRL users,
sponsors and other interested people about happenings at SSRL. SSRL is a
national synchrotron user facility operated by Stanford University for the
U.S. Department of Energy Office of Basic Energy
Sciences. Additional support for
the structural biology program is provided by
the DOE
Office of Biological and Environmental Research, the NIH
National Center for Research Resources and the NIH Institute for General Medical
Sciences. Additional information about
SSRL and its operation and schedules is available from the SSRL WWW
site.
__________________________________________________________________________
To leave the SSRL-HEADLINES distribution, send email as shown below:
To: LISTSERV@SSRL.SLAC.STANFORD.EDU
Subject: (blank, or anything you like)
The message body should read
SIGNOFF SSRL-HEADLINES
That's all it takes. (If we have an old email address for you that is
forwarded to your current address, the system may not recognize who
should be unsubscribed. In that case please write to
ssrl-headlines-request@ssrl.slac.stanford.edu and we'll try to figure out
who you are so that you can be unsubscribed.)
If a colleague would like to subscribe to the list, he or she should send
To: LISTSERV@SSRL.SLAC.STANFORD.EDU and use the message body
SUBSCRIBE SSRL-HEADLINES
4. Register for SSRL Summer Workshops in the
Structural Biology Sciences
July 28-30, 2006: Workshop on Small-Angle X-ray Scattering and Diffraction
Studies in Structural Biology. Organized by Hiro Tsuruta, Thomas Weiss and
Marc Niebuhr (SSRL), this workshop will provide hands-on training on
experimental techniques, software tutorial sessions primarily for solution
x-ray scattering studies, and talks on recent applications. Several shifts of
beam time have been allocated for short periods of data collection by workshop
participants. Register at:
http://www-ssrl.slac.stanford.edu/conferences/workshops/saxs2006/index.php
August 4, 2006: SSRL Crystallography Remote Access Workshop. There are still a
few slots remaining for this workshop which will be held at the
Hauptman-Woodward Medical Research Institute in Buffalo, NY on August 4.
Organizers Aina Cohen and Clyde Smith (SSRL), and Edward Snell (HWI) are
planning an agenda that will start with lectures and a live demonstration of
remote access data collection followed by two hands-on training sessions.
Attendees will learn about the beam line and software developments and how to
successfully complete all stages of a remote access experiment.
For more information and to register see:
http://smb.slac.stanford.edu/public/news/workshops/SSRL-HWI-2006/

5. Call for Nominations for Klein, Spicer and
Lytle Awards
http://www-ssrl.slac.stanford.edu/spiceraward.html
6.
Stanford Board of Trustees Committee Visits SLAC
The Committee on
Academic Policy, Planning and Management of the Stanford Board
of Trustees visited SLAC on Wednesday, June 14. After hearing an overview of
the SLAC scientific program by SLAC Director Jonathan Dorfan and remarks by
KIPAC Director Roger Blandford on the Particle Astrophysics and Cosmology
Program, they toured the site, including brief stops at three SSRL beam lines
where they heard from Keith Hodgson, Jennifer Leisch and Gordon Brown about the
role of synchrotron-related science in pharmaceutical drug discovery, research
towards a hydrogen economy and efforts aimed at restoring our polluted
environment, respectively.

7.
Secretary of Energy Bodman Speaks to DOE Employees
On Wednesday, June 14, Department of Energy Secretary Samuel Bodman reached out
to all 116,000 Department of Energy (DOE) federal and contractor employees via
live broadcast to express his appreciation for the work behind a number of
remarkable DOE achievements over the past year and to discuss future challenges
and opportunities within the organization. Secretary Bodman also stressed the
importance of a safe workplace. For a full transcript of his remarks see:
http://today.slac.stanford.edu/a/2006/06-16.htm

Secretary Bodman
8.
Summer Public Science Lectures at SLAC and Stanford University
Kavli Institute for Particle Astrophysics and Cosmology (KIPAC) astrophysicist
Sarah Church will give a SLAC public lecture, "Whispers of the Big Bang", on
Tuesday, June 27, at 7:30 p.m. in the SLAC Panofsky Auditorium.

The Thinker, 1880-1881. Promised gift to the Iris & B. Gerald
Cantor Center for Visual Arts at Stanford University
http://today.slac.stanford.edu/a/2006/06-16.htm
On Tuesday, August 29, Sean Brennan (SSRL) will give the next lecture in the
series on "A Comet on Earth: Results from the Stardust Mission."
Uwe Bergmann (SSRL) will give a talk titled "Archimedes: Ancient Text Revealed
with X-ray Vision on Thursday, August 3, as part of a series of outdoor science
lectures held this summer at the Stanford Cantor Arts Center. Parking, museum
entrance and lectures are free. Visitors can explore the Cantor Center starting
at 5 p.m. and then migrate outside to hear scientists talk in lay terms about
their research starting at 7 p.m. For the full schedule and directions see:
http://news-service.stanford.edu/news/2006/june21/summer-062106.html
http://museum.stanford.edu/visit/visit_MapDirections.html
9.
More SSRL News for a Wider Audience
http://today.slac.stanford.edu/subscribe.asp
Cool Running for SSRL. As SSRL prepares to turn up the juice to 500 mA,
engineers are busily upgrading components to handle the increased power. Key
among these components are the monochromators, devices that allow researchers
to fine-tune the x-ray beams to the needs of the experiment. Read more at
http://today.slac.stanford.edu/feature/monochromators.asp
Beam Line 7-2
monochromator.
Why the Aluminum Foil? Perhaps you've noticed that physicists seem to love
aluminum foil. Give them a high-precision, expensive vacuum chamber and what do
they do with it? Wrap the whole thing like leftovers. The real story, of
course, is more complicated than an arbitrary love for shiny things. Foil is
used for many things in the lab, and it turns out that when it comes to vacuum
chambers, aluminum foil is crucial to developing an ultra-high vacuum. Read
more at
http://today.slac.stanford.edu/feature/tin-foil.asp
10.
Macromolecular Crystallography Proposals due July 1
(contact:
L. Dunn, lisa@slac.stanford.edu)
http://www-ssrl.slac.stanford.edu/photonscience/jobs.html
SSRL Welcome
Page | Research
Highlights | Beam Lines | Accel
Physics
User
Admin | News & Events |
Safety Office

