
Contents of this Issue:
1. Science Highlight —
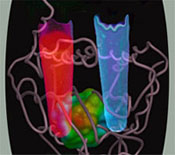
Revealing the Structure of a Hereditary Disease
(contact:
C.S. Raman, c.s.raman@uth.tmc.edu)

| ||
| Urine from HCP patients assumes an intense red fluorescence (left) when exposed to long-wavelength UV light and indicates the presence of coproporphyrin III. Normal urine (right) does not show this. |
2.
New Information in the Fight against Drug-resistant Bacteria
(contact: G. Chang, gchang@scripps.edu)
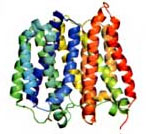 |
|
| A side view of the bacterial protein EmrD. |
3.
Beam Line 7 Gets a Makeover
(contacts:
T. Rabedeau@slac.stanford.edu;
D. Van Campen, campen@slac.stanford.edu)
 |
| 1st light in the new 7-3 hutch. |
Shortly thereafter a new BL7 began to emerge from the ashes of the old. In a
previous summer shutdown the old eight-pole electromagnetic wiggler and front
end were replaced by a modern 20-pole, 2.0-T hybrid wiggler and a 500-mA
capable front end. With the decommissioning of the old beam line equipment
complete it was time to upgrade the remainder of the beam line hardware. While
new hutches took shape outside the SPEAR3 concrete shield wall, the portion of
the beam line inside the SPEAR3 shielding received new masks, slits, and
water-cooled mirror systems. Shielding was augmented for 500-mA SPEAR3
operations and full power injection. By the end of the summer down all the new
beam line hardware located inside the SPEAR3 shielding enclosure was installed,
aligned, and function tested. As SPEAR3 started up for the fall run, the
installation and alignment of the optics contained in the beam transport hutch
commenced. Yet more masks and slits, a new focusing monochromator for BL7-1,
new LN-cooled monochromators for BL7-2 and BL7-3, new hutch stoppers, and even
more shielding appeared in the BL7-0 transport hutch. Over the winter shutdown
the new electrical power infrastructure for the beam line was connected to the
grid and instrumentation and control electronics installations began in
earnest.
Finally, following function checks in March and April, the LN monochromators
were cooled. Shortly thereafter BL7 x-ray commissioning commenced with BL7-3 on
May 3. Over the course of a few hours beam was conducted through the LN
monochromator and made its appearance in the BL7-3 experimental hutch. This
first light was followed by further commissioning of the BL7-3 monochromator
and collimating mirror system. By May 15 attention turned to the BL7-2 optics
commissioning followed by BL7-1 on May 19. By May 26 the first round of
monochromator and mirror commissioning activities on all three branch lines was
complete and light had been conducted into all three experimental hutches. The
coming weeks will see additional optics characterization and configuration
development activities followed by the first commissioning data collection
runs. For the users of the old BL7 it has been a bit of a wait, but your
patience will be rewarded shortly with three operational state-of-the-art
experimental stations illuminated by a modern insertion device and optics.
SSRL's SMB Bio-SAXS/D team will hold an on-site workshop on Small-Angle X-ray
Scattering and Diffraction Studies in Structural Biology on July 28-31, 2006.
This 4-day workshop will focus on the experimental aspects of non-crystalline
diffraction techniques in biology which complement high resolution structural
studies by crystallography, NMR and cryo-EM. The workshop will provide
hands-on training on experimental techniques and software tutorial sessions
primarily for solution x-ray scattering studies. Several shifts of beam time
have been allocated for short periods of data collection by workshop
participants. The latest advances in x-ray scattering and diffraction studies
on biological systems will be described by several experts in a diverse
spectrum of structural biology benefiting from non-crystalline diffraction
studies. Also planned are presentations on complementary experimental
approaches and modeling techniques. Participants will receive updates on
current and future developments at SSRL BL4-2, the dedicated small angle
scattering/diffraction facility for structural biology, funded by NIH NCRR and
DOE BER.
The first annual Workshop on Synchrotron X-ray Scattering Techniques in
Materials and Environmental Sciences was held at SSRL on May 16 and 17, 2006.
The aim of this workshop was to provide practical knowledge in x-ray scattering
methods with an emphasis on information that cannot be found in text books.
More than 60 researchers, mostly graduate students and postdocs, attended the
workshop. The first day consisted of introductory lectures on x-ray
diffraction, how to get the most data out of your beam time, and how to apply
various techniques. The second day involved "on-the-experiment" training at
four of SSRL's beam lines (1-4, 2-1, 11-3, and 10-2), with those attending (the
sessions were oversubscribed) gaining valuable experience from these
demonstrations for future beam time on their own proposals.
Based on the comments received, the workshop was tremendously successful with
one student remarking "this is the best workshop I have ever attended", and the
attendees came away with new knowledge about how to efficiently collect data at
SSRL's scattering beam lines. Copies of all the talks have been posted at:
We are presently deciding on the topics for next year's Synchrotron X-ray
Scattering Workshop and would appreciate comments from the user community.
July 1, 2006 is the next deadline for submitting macromolecular crystallography
proposals. SSRL's current experimental run ends at 6 a.m. on Monday, August 7.
Proposals submitted in July will be eligible for beam time when SSRL user
operations resume in early November 2006. For more information see Proposal
Submittal and Scheduling Procedures for Macromolecular Beam Lines at SSRL.
http://www-ssrl.slac.stanford.edu/users/user_admin/guide.html
A number of positions are currently available at the LCLS, LUSI and SSRL.
Please refer to the Photon Science Job Openings page for more information about
these job opportunities.
__________________________________________________________________________
SSRL Headlines is published electronically monthly to inform SSRL users,
sponsors and other interested people about happenings at SSRL. SSRL is a
national synchrotron user facility operated by Stanford University for the
U.S. Department of Energy Office of Basic Energy
Sciences. Additional support for
the structural biology program is provided by
the DOE
Office of Biological and Environmental Research, the NIH
National Center for Research Resources and the NIH Institute for General Medical
Sciences. Additional information about
SSRL and its operation and schedules is available from the SSRL WWW
site.
__________________________________________________________________________
To leave the SSRL-HEADLINES distribution, send email as shown below:
To: LISTSERV@SSRL.SLAC.STANFORD.EDU
Subject: (blank, or anything you like)
The message body should read
SIGNOFF SSRL-HEADLINES
That's all it takes. (If we have an old email address for you that is
forwarded to your current address, the system may not recognize who
should be unsubscribed. In that case please write to
ssrl-headlines-request@ssrl.slac.stanford.edu and we'll try to figure out
who you are so that you can be unsubscribed.)
If a colleague would like to subscribe to the list, he or she should send
To: LISTSERV@SSRL.SLAC.STANFORD.EDU and use the message body
SUBSCRIBE SSRL-HEADLINES
4. Electrical Safety Month across the
DOE Complex
(contact: I. Evans,
evans@slac.stanford.edu)
The month of May has been designated by the Department of Energy as Electrical
Safety Month. In response, SLAC Today, in partnership with the SLAC Electrical
Safety Committee, has been running a series of short articles to clarify the
electrical safety program at SLAC, promote awareness of electrical hazards and
to present electrical safety tips. These articles have covered a wide range of
topics including the proper use of personal protective equipment (PPE), lock
out tag out lock procedures, safe power strip and grounding practices, cable,
electrical equipment and circuit breaker safety.
In light of the serious electrical arc flash accident at SLAC in 2004, and
another recently at Brookhaven National Laboratories, it's important to note
that both accidents involved injuries which would have been significantly less
severe if correct warning labels had been posted and the workers had worn the
proper Personal Protective Equipment. Consequently, labels indicating the
hazard rating and a list of PPE required for resetting a breaker are appearing
on breaker panels all over SLAC. Only qualified workers with the permission of
their supervisor and training in electric shock and arc flash hazards are
allowed to reset breakers.

Also of particular interest to staff and users - a new Electrical Equipment
Inspection Program (EEIP) has been implemented at SLAC to ensure that custom or
modified electrical equipment does not shock, burn or catch fire when used
properly. All electrical equipment not tested and/or inspected by a nationally
recognized testing laboratory (NRTL) like Underwriters Laboratory (UL) must be
inspected on-site prior to use.
For more details on these electrical safety articles see:
http://today.slac.stanford.edu/a/,
http://www-group.slac.stanford.edu/essg/shorts.htm
David Thomassen, Acting Associate Director of the Office of Biological &
Environmental Research (BER), visited SLAC on Tuesday, May 2, and was given a
tour of SSRL's experimental floor. As the Acting Associate Director, David
oversees research activities including the Genomics: GTL program, low dose
radiation research, climate change research, environmental remediation
research, medical sciences research and user facilities for genomics, including
high throughput DNA sequencing, environmental molecular science, climate change
and structural biology.

Clay Sell (third from left) at the Overlook with Keith Hodgson, Nancy Sanchez,
John Galayda, Jonathan Dorfan, and Jeff Logan.
On Monday, May 15, SLAC hosted Clay Sell, Deputy Secretary of Energy, for
discussions with senior management, researchers and members from the Stanford
Site Office as he toured the facilities. For more on his visit see:
http://today.slac.stanford.edu/a/2006/05-16.htm
6.
Spring SLAC Policy Committee Meeting
(contact: K.O. Hodgson, hodgson@ssrl.slac.stanford.edu)
The meeting of the SLAC Policy Committee in early May focused on intermediate
and long range strategic planning for both the Photon Science and Particle and
Particle Astrophysics components of the laboratory. A perspective was provided
by Jonathan Dorfan, followed by more detailed plans from Keith Hodgson and
Persis Drell. The Committee heard a report of the National Academy EPP2010
decadal study from the committee chair, Harold Shapiro. SLAC's plans in the
PPA area align well with the recommendations of the Committee. The SPC also
heard an interim report from the chairperson of the National Academy AMO2010
decadal study and here LCLS science will align with priorities in the report.
The SPC had an in-depth discussion of the faculty development plan for Photon
Science that has been constituted to reflect strong growth in coming years due
to the investments in SPEAR3 and LCLS and the strengthening of centers of
scientific excellence. The ES&H Advisory Committee (ESHAC) reported out
formally to the SPC on several areas it had investigated, among them being the
user safety program at SSRL. The found the program to be very effective.
7.
Workshop on Small-Angle X-ray Scattering and Diffraction Studies in
Structural Biology
(organizers: Hiro Tsuruta, tsuruta@slac.stanford.edu;
Thomas Weiss, weiss@slac.stanford.edu;
Marc Niebuhr, neibuhr@slac.stanford.edu)
http://www-ssrl.slac.stanford.edu/conferences/workshops/saxs2006/index.php
8.
SSRL Remote Access Workshop to be Held in New York
(organizers: Aina Cohen, acohen@slac.stanford.edu;
Clyde Smith, csmith@slac.stanford.edu;
Edward Snell, esnell@hwi.buffalo.edu)

Complete macromolecular crystallography experiments are now routinely being
carried out at SSRL from remote locations, anywhere in the world, using secure
protocols. To showcase this unique capability the SSRL SMB Macromolecular
Crystallography group is taking its Remote Access Workshop on the road. This
workshop will be held at the Hauptman-Woodward Medical Research Institute in
Buffalo, NY on August 4. Organizers Aina Cohen and Clyde Smith (SSRL), and
Edward Snell (HWI) are planning an agenda that will start with lectures and a
live demonstration of remote access data collection in the morning followed by
two hands-on training sessions in the afternoon. Attendees will learn about
the beam line and software developments and how to successfully complete all
stages of a remote access experiment. The workshop is intended to be highly
practical with topics covered including:
For more information and to register see:
http://smb.slac.stanford.edu/public/news/workshops/SSRL-HWI-2006/
9.
Wrap-up on First Annual Workshop on Synchrotron X-ray Scattering
Techniques in Materials and Environmental Sciences
(organizers: J. Bargar, bargar@slac.stanford.edu;
M. Toney, mftoney@slac.stanford.edu)
http://www-ssrl.slac.stanford.edu/conferences/workshops/scatter2006/about.php.

10.
Macromolecular Crystallography Proposals due July 1
(contact:
L. Dunn, lisa@slac.stanford.edu)
http://www-ssrl.slac.stanford.edu/photonscience/jobs.html
SSRL Welcome
Page | Research
Highlights | Beam Lines | Accel
Physics
User
Admin | News & Events |
Safety Office