Porphyrins have earned the title "pigments of life" (1)
because they are essential for all life on planet earth. Porphyrins are the
precursors of heme, chlorophyll, and cyanocobalamin (vitamin B12).
Heme is an iron-coordinated porphyrin and serves as a prosthetic group in
several proteins to mediate catalysis (cytochromes, peroxidases) or recognize
diatomic molecules like oxygen (globins), carbon monoxide (CO) (cytochrome
oxidase), and nitric oxide (NO) (soluble guanylyl cyclase) (2,3). With the exception of a few microorganisms,
heme is found in all three archaea, prokarya, and eukarya kingdoms (2). On the one hand, heme biosynthesis is a sine qua non
for the function of heme-containing enzymes and proteins. For example, gaseous
messengers like NO cannot be biosynthesized in humans without the
heme-containing enzyme nitric oxide synthase (4). On the
other hand, enzymatic degradation of heme results in the generation of CO - a
key cellular signal generated as a by product of heme oxygenase chemistry.
| |
 |
|
Figure 1.
Urine from HCP patients assumes an intense red fluorescence (left) when
exposed to long-wavelength UV light and indicates the presence of
coproporphyrin III. Normal urine (right)does not show this.
|
Porphyrias are a group of inborn errors of heme biosynthesis that are
designated as hepatic or erhthropoietic based on clinical manifestation and the
primary site in which the enzymatic defect manifests (5).
For example, the penultimate step of the heme biosynthesis involves the enzyme
protoporphyrinogen oxidase. Defects in this enzyme lead to variegate porphyria
and roughly 10,000 South Africans suffer from this disease. Based on
genealogical evidence it has been shown that this disease was inherited from a
single individual - a woman who emigrated from the Netherlands in 1688. As a
result, all the South African families with variegate porphyria exhibit the
same substitution (R59W) in the protoporphyrinogen oxidase gene (6). The madness suffered by King George III (1738 - 1820) has also
been attributed to hereditary porphyria (7).
Hereditatry coproporphyria (HCP) is an autosomal dominant acute hepatic
porphyria with incomplete penetrance due to half-normal activity of
coproporphyrinogen oxidase (CPO) (8) Defects in this enzyme
result in acute attacks characterized by severe abdominal pain, hypertension,
tachycardia, and neurologic dysfunction. In some cases skin photosensitivity
is also seen. In the absence of prompt and appropriate treatment, HCP can very
rapidly become a life-threatening medical emergency. CPO catalyzes the
antepenultimate step in heme biosynthesis. First purified in the early 1960s,
CPO mediates the oxidative decarboxylation of propionic acid side chains of
rings A and B in coproporphyrinogen III without utilizing transition metals,
reducing agents, thiols, prosthetic groups, organic cofactors, or modified
amino acids (9). Whereas the stereochemistry of this reaction
has been worked out, the molecular oxygen consumption poses an interesting
chemical puzzle.
 |
|
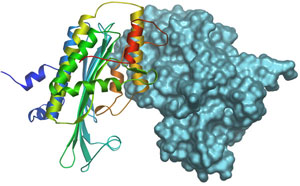
Figure 2.
Crystal structure of the CPO dimer. One of monomer is shown as a ribbon and the
second monomer with surface representation.
|
We have solved the crystal structure of human CPO at 1.58 Ĺ resolution (Fig. 2)
and show that it is a dimer in the native state (10). CPO
has a novel tertiary topology with an unusually flat seven-stranded b-sheet surrounded by a-helices. To our great surprise, we found a molecule of
citrate (tricarboxylate) tightly bound at the active site. By comparing the
interaction of citrate in CPO and the structurally unrelated aconitase, we have
identified the key catalytic residues. Furthermore, we have proposed two
models for how this enzyme catalyzes the successive oxidative decarboxylation
reactions. The first model involves a radical intermediate whereas as the
second proceeds via carbanion formation.
This project was funded by the Pew Charitable Trusts via a Pew Scholar Award,
the Robert A. Welch Foundation and by a grant from the Ministry of Education,
Youth, and Sports of the Czech Republic.
Primary Citation
Lee DS, Flachsova E, Bodnarova M, Demeler B, Martasek P, Raman CS. Structural
basis of hereditary coproporphyria. Proc Natl Acad Sci USA. 2005 Oct
4;102(40):14232-7.
References
-
Battersby AR, Fookes CJ, Matcham GW, McDonald E. Biosynthesis of the
pigments of life: formation of the macrocycle. Nature. 1980 May
1;285(5759):17-21.
-
Panek H, O'Brian MR. A whole genome view of prokaryotic haem
biosynthesis. Microbiology. 2002 Aug;148(Pt 8):2273-82.
-
Nioche P, Berka V, Vipond J, Minton N, Tsai A-L, Raman CS. Femtomolar
Sensitivity of a NO Sensor from Clostridium botulinum. Science.
2004 Nov 306:1550-53.
-
Raman CS, Martásek P, Masters BSS. Structural Themes Determining
Function in Nitric Oxide Synthases. In The Porphyrin Handbook, Kadish KM, Smith
KM, Guilard R, Eds. (New York: Academic Press), Vol 4: Biochemistry and
Binding: Activation of Small Molecules pp. 293 - 340 (2000)
-
Badminton MN, Elder GH. Molecular mechanisms of dominant expression in
porphyria. J Inherit Metab Dis. 2005;28(3):277-86.
-
Meissner PN, Dailey TA, Hift RJ, Ziman M, Corrigall AV, Roberts AG,
Meissner DM, Kirsch RE, Dailey HA. A R59W mutation in human
protoporphyrinogen oxidase results in decreased enzyme activity and is
prevalent in South Africans with variegate porphyria. Nat Genet. 1996
May;13(1):95-7.
-
Cox TM, Jack N, Lofthouse S, Watling J, Haines J, Warren MJ. King
George III and porphyria: an elemental hypothesis and investigation. Lancet.
2005 Jul 23-29;366(9482):332-5.
-
Martasek P. Hereditary coproporphyria. Semin Liver Dis.
1998;18(1):25-32.
-
Sano S, Granick S. Mitochondrial coproporphyrinogen oxidase and
protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173-80.
|
| PDF Version | | Lay Summary
|
| Highlights
Archive |
|
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences.
The SSRL Structural Molecular Biology Program is supported by the Department of
Energy, Office of Biological and Environmental Research, and by the National
Institutes of Health, National Center for Research Resources, Biomedical
Technology Program, and the National Institute of General Medical Sciences.
|
|
|