 |
|
The group solved the structure of a complex of Myosin A tail interacting protein (MTIP) and MyoA-tail to 2.6 Å. The crystallized samples used contained three different conformations of MTIP in one single crystal, which allowed the scientists to assess the protein's crucial interaction with the MyoA-tail by comparing three independent subunits. MTIP bridges between the membranes associated proteins and the tail of myosin, which interacts with short actin filaments. The actin filaments are attached to the enzyme aldolase, which connects actin and Thrombospondin Anonymous Repeat Protein (TRAP), which initiates the process of invasion.
The researchers performed inhibition experiments with MyoA-tail to test the viability of P. falciparum, a dangerous malaria-causing Plasmodium species. Because the MyoA-tail interacting P. falciparum MTIP residues differ significantly from the human homolog, structure-based drug design can exploit this feature and may hold promise for new therapies to combat malaria infection. The results were published in the March 28, 2006 edition of the Proceedings of the National Academy of Sciences.
To learn more about this research see the full scientific highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/MTIP.html
2. Science Highlight —
On the Role of Copper Regulation in a M. tuberculosis Repressor
(contact:
G.N. George, g.george@usask.ca)
Scientists have discovered a gene for a protein that regulates the cellular response to copper in the bacterium that causes tuberculosis. These findings, reported in the January issue of Nature Chemical Biology, explain how a wide variety of bacteria control copper concentrations within their cells, and this understanding could lead to new treatments for tuberculosis.
The team discovered the gene that encodes a "Copper-sensitive operon Repressor" (CsoR), which controls the production of copper-binding proteins and is present in many types of bacteria. X-ray absorption spectroscopy (XAS), measured mainly on SSRL's beam line 9-3, on purified samples of the copper-binding protein from M. tuberculosis was used to discern the chemical mechanism behind the copper-binding protein.
Previous work had shown that CsoR was part of a cluster of genes active in M. tuberculosis infecting the lungs of mice. Analysis of the DNA sequence of a nearby gene led researchers to hypothesize that it encoded a protein that acts as a "copper pump" that drives excess copper out of the cell, and that CsoR was the critical regulator of this process.
 | |
|
| |
To learn more about this research see the full scientific highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/CsoR.html
3. Science Highlight —
Minding the Gaps: Explaining the Behavior of a High-Temperature Superconductor
(contacts:
K. Tanaka, ktanak@stanford.edu; W.S. Lee, leews@stanford.edu;
D.H. Lu, dhlu@stanford.edu;
Z.-X. Shen, shen@ssrl.slac.stanford.edu)
Scientists at Stanford University have recently made an important discovery about the coexistence of two distinct energy gaps in photoemission spectra of high temperature superconductors. The two gaps have opposite doping dependence, which provides an explanation for the contradictory results about the superconducting gap deduced from different experimental techniques. The findings, published in the December 22 issue of Science, have profound implications for the mechanism of high temperature superconductivity.
The researchers studied the effects of changes in doping level on the evolution of the electronic structure in the highly underdoped cuprate superconductor Bi2Sr2Ca1-xYxCu2O8+d (Bi2212), using powerful angle-resolved photoemission spectroscopy (ARPES). Significantly
 | |
| The symmetrized spectra at (A) the tip of the Fermi Arc region and (B) the antinodal region. Their corresponding locations on the Fermi surface are shown in the inset of (A). The shaded area denotes the region inside the gap. (C) Doping dependence of the gap magnitude on various locations along the Fermi Arc region and in the antinodal region with their locations shown in the inset together with Tc. The dashed line indicates the pseudogap at the antinodal region reported by previous ARPES studies on Bi2212 system. [larger view] | |
Based on their experimental observations of two coexisting energy gaps, the group, led by Professor Zhi-Xun Shen, proposed a scenario with two important implications. First, the pseudogap state in the deeply underdoped samples probably competes with the superconducting state rather than preceding it, as previously suggested. Second, the data suggest that the weakened superconductivity in the underdoped regime arises not only from the loss of phase coherence but also from the weakening of electron pairing amplitude because of competing states. The implications of these findings could lead to a microscopic theory of high-temperature superconductivity.
To learn more about this research see the full scientific
highlight at:
http://www-ssrl.slac.stanford.edu/research/highlights_archive/pseudogap.html
The Structural Molecular Biology BioXAS group will host an X-ray Absorption
Spectroscopy (XAS) Short Course at SSRL on March 13-16, 2007. The training will
include two days of lectures which will cover basic theory, experimental
considerations, and applications. The lectures will be followed by two days of
rotating practical sessions, which will include hands-on data collection at the
beam line and data analysis. Participants are encouraged to bring their own
samples to test feasibility for future data collection. Space will be limited
to 16 participants, so early application is encouraged and will be available
soon through the SSRL website. For more information, please contact Serena
DeBeer George (serena@slac.stanford.edu).
With funding from NIH, SSRL is developing a hard x-ray, full field imaging
facility on beam line 6-2 capable of 20-50 nm resolution (depending on the
particular imaging mode) with tomographic and phase contrast capabilities. The
facility is being built around an Xradia nanoXCTT S-60 (Synchrotron Tomographic
Imaging X-Ray Microscope) which was delivered in November 2006 and installed in
a new hutch at the end of beam line 6-2. The first commissioning run took place
during a 10-day period in December in which we successfully demonstrated a
spatial resolution of better than 50 nm in a 10 micrometer field of view using
both absorption and phase contrast. The first image was of a test pattern
consisting of a radial set of tapered gold lines with minimum feature sizes of
50 nm. Although the initial exposures were carried out at 8 keV, the microscope
is able to image photons in the range of approximately 5-14 keV allowing us to
perform spectroscopic imaging of a wide range of elements relevant to both the
life and physical sciences. The high brightness available from beam line 6-2
resulted in exposure times below 1 second which allowed us to obtain the
exposures needed for a tomographic reconstruction within a few minutes. We will
continue the commissioning process over the next several months, carrying out
imaging at a spatial resolution of 20 nm and imaging a wide variety of samples.
Please contact Piero Pianetta (pianetta@stanford.edu) if you want to learn more
about this instrument or have samples that might be of interest for imaging.
The tools of the trade for x-ray researchers are often collections of
instruments rather than a single, monolithic apparatus like a large microscope.
The microprobe at beam line 2-3 brings together several commonly used devices,
but does so in a way that gives researchers a suite of new capabilities,
optimized for conducting environmental research. Read more of this SLAC Today
article by Brad Plummer at:
http://today.slac.stanford.edu/feature/2007/beamline2-3.asp
A detailed summary of the workshop and copies of the presentations
can be found at
__________________________________________________________________________
SSRL Headlines is published electronically monthly to inform SSRL users,
sponsors and other interested people about happenings at SSRL. SSRL is a
national synchrotron user facility operated by Stanford University for the
U.S. Department of Energy Office of Basic Energy
Sciences. Additional support for
the structural biology program is provided by
the DOE
Office of Biological and Environmental Research, the NIH
National Center for Research Resources and the NIH Institute for General Medical
Sciences. Additional information about
SSRL and its operation and schedules is available from the SSRL WWW
site.
__________________________________________________________________________
To leave the SSRL-HEADLINES distribution, send email as shown below:
To: LISTSERV@SSRL.SLAC.STANFORD.EDU
Subject: (blank, or anything you like)
The message body should read
SIGNOFF SSRL-HEADLINES
That's all it takes. (If we have an old email address for you that is
forwarded to your current address, the system may not recognize who
should be unsubscribed. In that case please write to
ssrl-headlines-request@ssrl.slac.stanford.edu and we'll try to figure out
who you are so that you can be unsubscribed.)
If a colleague would like to subscribe to the list, he or she should send
To: LISTSERV@SSRL.SLAC.STANFORD.EDU and use the message body
SUBSCRIBE SSRL-HEADLINES
4. XAS Course for Structural Molecular
Biology Applications in March
(contact:
S. DeBeer George, serena@slac.stanford.edu)
5. New Hard X-ray Microscope Commissioned on
Beam Line 6-2
(contact:
P. Pianetta, pianetta@slac.stanford.edu)
http://xradia.com/Products/nanoxcts60.html
6.
Scanning the Microworld: SSRL's New Hard X-ray Microprobe
(contact:
S. Webb, samwebb@slac.stanford.edu
Toxins like arsenic occur in soil in many parts of the world, and certain
species of ferns are known for their ability to soak up these poisons. But
characterizing just how they do it can be a tricky process. Now, thanks to a
new microprobe at SSRL's beam line 2-3, unlocking the secrets of how plants
collect and store arsenic is getting both easier and more accurate.

Researchers have long used x-rays as a tool for studying environmental
contaminants. Microprobes enable researchers to peer into the chemical
structure of materials and map out the locations of compounds within a sample,
such as where contaminants adhere to grains of soil. Unlike other x-ray
techniques that look only at overall compositions of a sample, the microprobe
allows a sample to be moved around, creating detailed picture of where
compounds lie. As the x-rays scan point by point, an image emerges that shows
precisely where each elements of interest resides.
7. Sagittal Focusing, LN-cooled Monochromator
Installed on Beam Line 7-2
(contact:
D. Van Campen, campen@slac.stanford.edu)
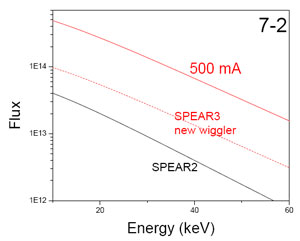
As part of the BL7 500mA upgrade, SSRL staff designed and assembled a sagittal
focusing, LN-cooled monochromator to facilitate focused beam experiments at
higher energies than typically accommodated by mirror optics. During the first
two weeks of December the standard LN-cooled monochromator temporarily
installed on BL7-2 was replaced by the prototype sagittal focusing
monochromator. Commissioning of this monochromator commenced on December 20
with the observation of first monochromatic light. Over the next couple days
focused beam was developed over an initial 6-15 keV energy range. Typical focus
spot full-width-at-half maximum was approximately 0.5 mm horizontal by 0.15 mm
vertical. Some residual focusing crystal strain was identified, that precluded
obtaining this focus over the full horizontal acceptance of the beam line.
There is an ongoing effort to characterize and eliminate the sources of this
strain. By using a somewhat reduced horizontal acceptance, the beam line will
be put into full operations beginning in February.

Beam
Line 7-2 sagittal focusing, LN-cooled monochromator.
[larger view]
8.
Workshop on New Directions in X-ray Scattering
(contacts: A. Mehta,
mehta@slac.stanford.edu; M.F. Toney, mftoney@slac.stanford.edu)
On December 6, 2006, SSRL held a day-long workshop to solicit
user input on the new directions that the SSRL materials science scattering
program should take to better meet the needs of materials and chemical
sciences, upgrade beam lines to take advantage of modern instrumentation and
maximally utilize the improved source characteristics that the SPEAR3 upgrade
provides. The workshop concluded that most of the needs can be met by two
wiggler and one bend magnet beam lines (upgraded or rebuilt BLs, 10-2, 7-2 and
2-1). The workshop was held on the Stanford University main campus and was
attended by about 30 participants, from industry, national labs, and
universities.
http://www-ssrl.slac.stanford.edu/conferences/workshops/newdirections2006/index.php
9.
Photon Science Job Opportunities
A number of positions are currently available at SSRL, LUSI and LCLS.
Please refer to the Photon Science Job Openings page for more information about
these job opportunities.
http://photonscience.slac.stanford.edu/jobs.php
SSRL Welcome
Page | Research
Highlights | Beam Lines | Accel
Physics
User
Admin | News & Events |
Safety Office

