| People |
| BL 11-2 |
| Reports &Publications |
| Model Compound Library |
| SixPACK |
| Glitch Curves |
| MES User Resources & Instrumentation |
| Environmental Remediation Science at SSRL |
| MEIS Home |
| SSRL |
| Stanford EMSI |
| SLAC |
The SSRL-BER Environmental Remediation Science program supports researchers in NABIR, EMSP, and BER-EMSI programs, funded by the DOE Office of Biological and Environmental Sciences (BER). We provide advice and direct support for planning and conducting experiments at SSRL and analyzing results. BER-ERSD supports similar programs at ALS, APS, and NSLS.
The mission of this program is to enable a deeper knowledge of the fundamental chemical, biological, and physical factors that govern the reactivity and cycling of contaminants in the environment. This work directly supports the development of new (bio)remediation technologies and the remediation of high level waste (HLW) and contaminated facilities.Scientists who are interested in information about experimental capabilities and/or conducting measurements at SSRL should contact Sam Webb or John Bargar. Additional information about SSRL is provided at the SSRL home page.
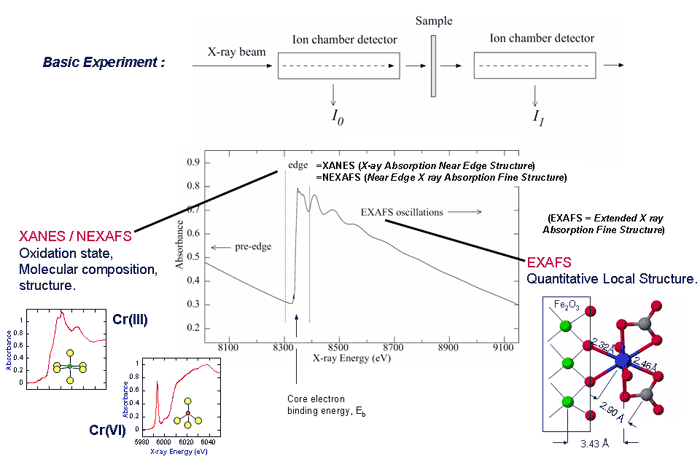
XAS and X-RAY SCATTERING TECHNIQUES and EXPERIMENTAL CAPABILITIES at SSRL.

This program is supported by DOE-BER, Environmental Remediation Sciences Division. Participants are asked acknowledge BER and other agencies’ support for SSRL on their publications.
Primary Contacts for Environmental Remediation Research at SSRL
|
|
 |
 |